Product Images Tamsulosin Hydrochloride
View Photos of Packaging, Labels & Appearance
- Figure 1 Mean Plasma Tamsulosin Hydrochloride Concentrations Following Single-Dose Administration of Tamsulosin Hydrochloride Capsules 0.4 mg Under Fasted and Fed Conditions (n=8) - tamsulosin fig1
- Figure 2A Mean Change from Baseline in Total AUA Symptom Score (0 to 35) Study 1 - tamsulosin fig2
- Figure 2B Mean Change from Baseline in Total AUA Symptom Score (0 to 35) Study 2 - tamsulosin fig3
- Figure 3A Mean Increase in Peak Urine Flow Rate (mL/Sec) Study 1 - tamsulosin fig4
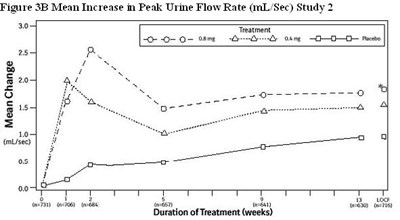
- Figure 3B Mean Increase in Peak Urine Flow Rate (mL/Sec) Study 2 - tamsulosin fig5
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.4 mg (100 Capsule Bottle) - tamsulosin fig6
- Chemical Structure - tamsulosin str
Product Label Images
The following 7 images provide visual information about the product associated with Tamsulosin Hydrochloride NDC 57237-014 by Rising Pharma Holdings, Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1 Mean Plasma Tamsulosin Hydrochloride Concentrations Following Single-Dose Administration of Tamsulosin Hydrochloride Capsules 0.4 mg Under Fasted and Fed Conditions (n=8) - tamsulosin fig1

Figure 2A Mean Change from Baseline in Total AUA Symptom Score (0 to 35) Study 1 - tamsulosin fig2
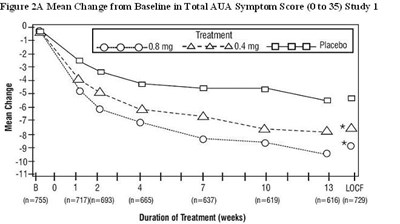
The image shows a graph depicting the mean change from baseline in the total American Urological Association (AUA) symptom score ranging from 0 to 35, in which 0.08mg A and 0.4mg A doses and placebo treatments were administered over a duration of 1 to 12 weeks in study 1. The graph shows that the mean change in the symptom score decreased over time with both doses of A and placebo treatment. LOCF (Last Observation Carried Forward) method was used to account for missing data.*
Figure 2B Mean Change from Baseline in Total AUA Symptom Score (0 to 35) Study 2 - tamsulosin fig3
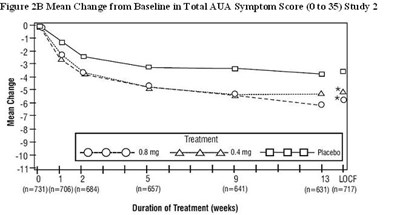
Figure 2B shows the mean change from baseline in the total American Urological Association Symptom Score (AUA) from Study 2. The graph displays the durations of treatment (in weeks) on the x-axis and the mean change in the symptom score (ranging from 0 to 35) on the y-axis. The three treatments compared are O=0O—0-08mg LNt, 0.4mg, and placebo. The data is based on the LOCF method and includes the number of patients (n) for each treatment group at various time points.*
Figure 3A Mean Increase in Peak Urine Flow Rate (mL/Sec) Study 1 - tamsulosin fig4

The figure shows the mean increase in peak urine flow rate (in L/sec) in Study 1. The treatment groups include 0.08 mg and 0.4 mg, as well as a placebo. The mean change in mL/sec is also depicted. The duration of treatment ranged from 7 to 13 weeks, and the sample sizes varied from 617 to 755.*
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.4 mg (100 Capsule Bottle) - tamsulosin fig6

This is a description of a medication, specifically Tamsulosin hydrochloride USP 0.4 mg, which is contained in a capsule labeled under NDC 57237-014-01. The recommended dosage can be found in the prescribing information, and the medication should be stored between 20-25°C. The container should be tight and controlled room temperature should be maintained. The medication is distributed by a company called Rising Health, LLC in Saddle Brook, NJ, and it was manufactured in India. The code listed is TS/DRUGS/22/2009, and the package contains 100 capsules, the most recent revision being in November 2017.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.