Product Images Galantamine
View Photos of Packaging, Labels & Appearance
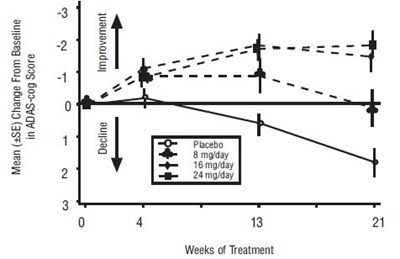
- Figure 1: Time-Course of the Change From Baseline in ADAS-cog Score for Patients Completing 21 Weeks (5 Months) of - galantamine fig1
- galantamine fig10
- galantamine fig11
- FIGURE 12 - galantamine fig12
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 mg (60 Tablets Bottle) - galantamine fig13
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 8 mg (60 Tablets Bottle) - galantamine fig14
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 12 mg (60 Tablets Bottle) - galantamine fig15
- galantamine fig2
- galantamine fig3
- galantamine fig4
- FIGURE 5 - galantamine fig5
- galantamine fig6
- galantamine fig7
- FIGURE 8 - galantamine fig8
- galantamine fig9
- Structure - galantamine str
Product Label Images
The following 16 images provide visual information about the product associated with Galantamine NDC 57237-050 by Rising Pharma Holdings, Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1: Time-Course of the Change From Baseline in ADAS-cog Score for Patients Completing 21 Weeks (5 Months) of - galantamine fig1
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 mg (60 Tablets Bottle) - galantamine fig13

This is a description for Galantamine Tablets, USP made in India consisting of 60 tablets. Each film-coated tablet has Galantaming hydrobromida USP and is equivalent to 4 mg galantamine bass. The recommended usual dosage is provided in the accompanying product literature. The tablets should be stored between 20° to 25°C (68°F to 77°F) with excursions permitted between 15°C to 30°C (59°F to 86°F). It is important to keep the tablets out of reach of children. The revision date for this product is 01/2018.*
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 12 mg (60 Tablets Bottle) - galantamine fig15

The text describes a medication called "Rising Nocs7257-051-50 Galantamine Tablets, USP". Each film-coated tablet contains 12 mg of galantamine hydrobromide USP. The product literature provides instructions to store the medicine between 20° to 25°C and keep it out of reach of children. The medication is distributed by Fising Health, LLC and was made in India. The description also includes a revision date of 01/2018 and a code of TS/DRUGS/10/1993. The text mentions an "over printing zone," but it is not clear what that refers to.*
FIGURE 8 - galantamine fig8
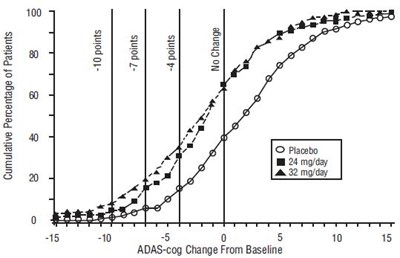
The text seems to be a graph or chart and it shows the Cumulative Percentage of Patients with no change in ADAS-cog score from Baseline. The X-axis has values ranging from -5 to 15 with increments of 5, indicating the ADAS-cog Change From Baseline. The Y-axis shows the percentage of patients. The chart has two lines, one representing the placebo group and the other showing 24 my/day or 32 my/day groups. No further information is available from the given text.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.











