Product Images Benefix
View Photos of Packaging, Labels & Appearance
Product Label Images
The following 37 images provide visual information about the product associated with Benefix NDC 58394-634 by Wyeth Biopharma Division Of Wyeth Pharmaceuticals Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure - benefix 22

This text provides information on the manufacturer of a product, specifically, Wyeth Pharmaceuticals LLC, which is a subsidiary of Pfizer Inc located in Philadelphia, PA 19101.*
benefix 22a

Benefix® is a medication that provides Coagulation Factor IX (Recombinant) and is manufactured by Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc. The medication is stored at room temperature and comes with a syringe for administration. The text provides storage instructions and information on reconstitution for Factor X deficiencies.*
benefix 22b

This is an instruction label found on a disposable syringe containing a diluent used for BeneFix. The diluent is sterile and nonpyrogenic, containing no preservatives. The main ingredient in the diluent is 0.234% sodium chloride. The manufacturer is Wyeth Pharmaceuticals with a subsidiary Pfizer Inc. The diluent is manufactured by Vetter Pharma-Fertigung GmbH & Co. KG in Ravensburg or Eisenbahnsirosse, Germany. There is a lot and expiration date provided on the label.*
benefix 22c

This is a packaging of a single-use vial containing 5 mL pre-filled diluent syringe with recombinant Coagulation Factor IX for replacement therapy. It is necessary to avoid freezing or any damage to the diluent syringe as it may cause adverse effects. The product comes with one sterilized prefilled syringe with a solution of 0.234% Sodium Chloride for infection, one gauze pad, a package insert for guidance, and a lot number. The storage condition is at room temperature, and the product does not contain plasma or albumin. The rest of the text appears to be non-English and unreadable, possibly a barcode or manufacturing details.*
benefix 22d

Coagulation Factor IX (Recombinant) is a medication manufactured and stored at room temperature by Vyeth Pharmaceuticals LLC. It is a subsidiary of Pfizer located in Philadelphia. It is approved by the US Government and should be stored between 2 and 30°C. The medication is intended for use as a treatment for Factor IX deficiency. For more information and dosage instructions, refer to the package insert labeled with PAA133668.*
benefix 22e

This is a description for a drug diluent for use with BeneFix, a recombinant coagulation factor X. The diluent is a 0.234% sodium chloride solution, provided in a sterile, nonpyrogenic 5mL disposable syringe. It contains no preservatives and is manufactured by Vetter Pharma-Fertigung GmbH & Co. KG in Ravensburg, Germany or Longenargen, Germany. The product is made for Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc., located in Philadelphia, PA. The LOT and EXP details are not available.*
benefix 22f

This package contains a single-use vial of BeneF-Coagulation Factor IX (Recombinant), ready for intravenous administration. The package includes one sterile pre-filled syringe of 0.234% Sodium Chloride solution for injection, with plunger rod, a sterile vial adapter reconstitution device, one sterile infusion set, two alcohol swabs, one bandage, one gauze pad, and one mkm insert. This medication should not be frozen, or damage to the diluent syringe may occur. The GTIN of this package is 0058304635000, and it is for Factor IX replacement therapy only. It must be stored at room temperature and has no plasma or albumin. The enclosed sterile profiled syringe of 0.234% Sodium Chloride should be used to reconstitute the medication.*
benefix 22g

This is a product description for a medication called BenefFix® SIGEOIDED. It is a recombinant Coagulation Factor IX used for Factor IX replacement. The manufacturer is Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc. The medication can be stored at room temperature and the given contact number is (01)00358394134031.*
benefix 22h

This is a description of a drug diluent called 0.234% Sodium Chloride Diluent, which is a sterile and non-pyrogenic solution that does not contain any preservatives. It is designed to be used with BeneFix® Coagulation Factor X (Recombinanf) and comes in a disposable syringe with a capacity of 5mlL y. The manufacturer of this diluent is Wyeth Pharmaceuticals, a subsidiary of Pfizer, with headquarters in Philadelphia, PA. The product is made by Vefter Pharma-Fertigung GmbH & Co. KG in Ravensburg or Longenargen, Germany, as indicated by the information provided on the packaging. The Lot and Exp numbers are not available.*
benefix 22i

This is a description of a medication called Coagulation Factor IX (Recombinant) used for Factor IX Replacement Therapy. It is stored at room temperature and comes with a prefilled syringe of 0.234% Sodium Chiorde for injection. The medication is not to be frozen as it may damage the syringe. Additionally, the package contains alcohol swabs, gauze pad, and a package insert. The text also includes various numeric and alphanumeric codes with no clear meaning or context.*
PRINCIPAL DISPLAY PANEL - 250 IU SINGLE USE VIAL LABEL - benefix 23
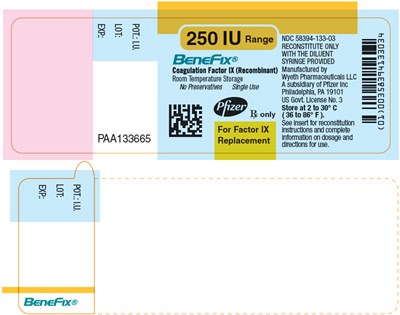
BenefFix® is a product that contains Coagulation Factor IX (Recombinant) and is manufactured in Philadelphia, PA. The product comes with a syringe and should be stored at room temperature between 2-30°C. The recommended dosage and instructions for use can be found on the insert or reconstitution. The NDC code for the product is 58394-133-03.*
PRINCIPAL DISPLAY PANEL - 250 IU Kit Carton - benefix 25

This description contains technical text related to the storage, administration, and use of the medical product "BeneFIX", which is a Coagulation Factor IX (Recombinant) used for factor IX replacement therapy only. The product is stored at room temperature, and it comes with one single-use vial with a 5-mL prefilled diluent syringe, a sterilized infusion set, alcohol swabs, a bandage, and a gauze pad. A package insert is included. The text includes warnings not to freeze the product and provides a GTIN (035630463030). The rest of the text is not readable.*
PRINCIPAL DISPLAY PANEL - 500 IU SINGLE USE VIAL LABEL - benefix 26
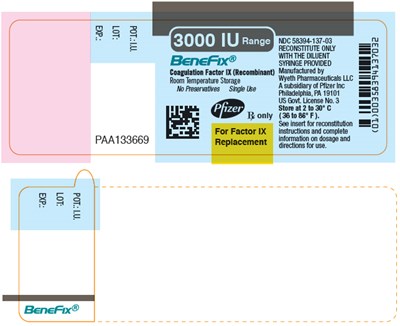
This is a description of a pharmaceutical product called BeneFix® by Wyeth Pharmaceuticals LLC. It is a recombinant Coagulation Factor IX used to treat blood clotting disorders. The storage temperature for the product is mentioned and directions for use are available.*
PRINCIPAL DISPLAY PANEL - 500 IU Kit Carton - benefix 27
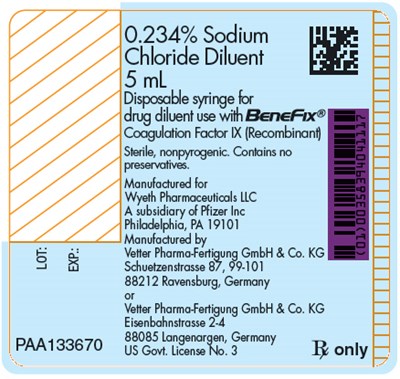
This is a description of a drug diluent containing 0.234% Sodium Chloride. It is a sterile and nonpyrogenic solution that comes in a 5mL disposable syringe without preservatives. The diluent is intended for use with BeneFix, a recombinant Coagulation Factor X medication. The manufacturer is Wyeth Pharmaceuticals LLC, a Pfizer subsidiary located in Philadelphia, PA, and the product is manufactured by Vefter Pharma-Fertigung GmbH & Co. KG, either at Schuetzenstrasse or Eisenbahnsirosse in Germany. The text also includes Lot and expiration dates.*
PRINCIPAL DISPLAY PANEL - 1000 IU SINGLE USE VIAL LABEL - benefix 28
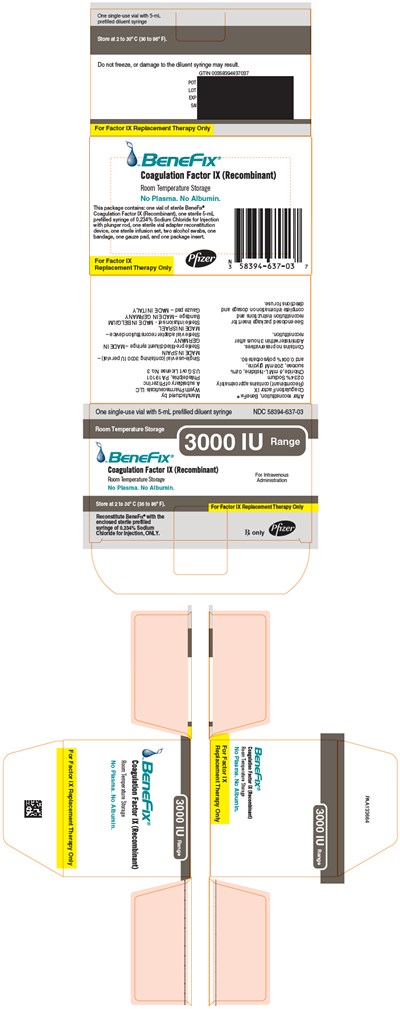
This is a package insert containing information about a product called Benefix® Coagulation Factor IX (Recombinant), which is used for intravenous administration. The package contains a single-use sterile prefilled diluent syringe with a 5 mL profiled volume. The product should be stored at room temperature and should not be frozen. The insert warns that freezing may result in damage to the diluent syringe. The package also includes a sterile pre-filled syringe of 0.234% sodium chloride for injection with plunger rod, sterile vial adapter reconstitution device, infusion set with two alcohol swabs, one bandage, one gauze pad, and package insert. The text appears to contain several typographical errors and unrecognizable characters.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.





















