FDA Label for Saline Nasal
View Indications, Usage & Precautions
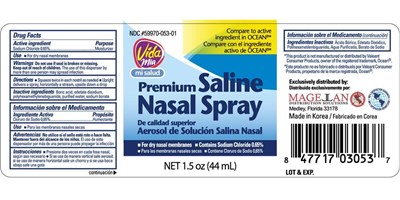
Saline Nasal Product Label
The following document was submitted to the FDA by the labeler of this product Navarro Discount Pharmacies, Llc. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Otc - Active Ingredient
Sodium Chloride 0.65%
Purpose
Moisturizer
Use
- For dry nasal membranes
Warnings
Do not use if seal is broken or missing.
Keep Out Of Reach Of Children
The use of this dispenser by more than one person may spread infection.
Directions
- Squeeze twice in each nostril as needed
- Upright delivers a spray, horizontally a stream, upside down a drop
Inactive Ingredients
Boric acid, edetate disodium, polyhexamethylenebiguanide, purified water, sodium borate
Package Label.Principal Display Panel
saline nasal spray
* Please review the disclaimer below.