Product Images Sephience
View Photos of Packaging, Labels & Appearance
- Box - Box
- Inner Label0 - Inner Label0
- Inner Label2 - Inner Label2
- Outer Label0 - Outer Label0
- Outer Label2 - Outer Label2
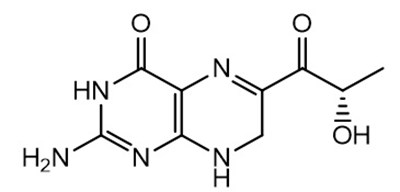
- Chemical Structure - sephience 01
- Figure 1 - sephience 02
- 250mg Dose Packet - sephience 03
- 1000mg Dose Packet - sephience 04
- Figure A - sephience 05
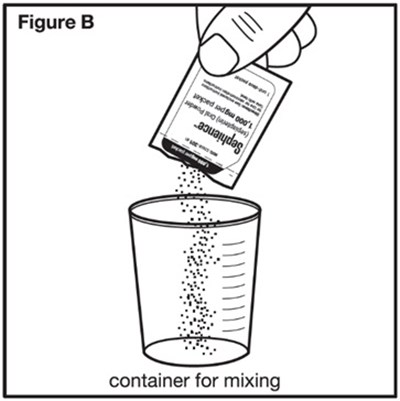
- Figure B - sephience 06
- Figure C - sephience 07
- Figure D - sephience 08
- Figure E - sephience 09
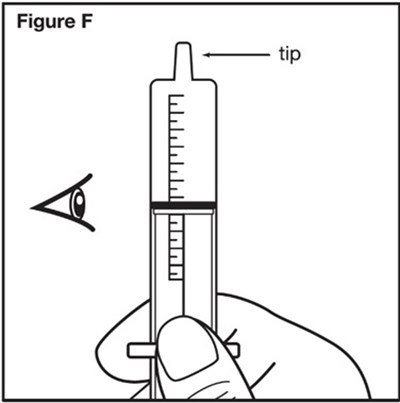
- Figure F - sephience 10
- Figure G - sephience 11
- Figure A1 - sephience 12
- 250mg foil - sephience 13
- 1000mg foil - sephience 14
- Figure B1 - sephience 15
- Figure C1 - sephience 16
- Figure D1 - sephience 17
Product Label Images
The following 22 images provide visual information about the product associated with Sephience NDC 60468-007 by Allphamed Pharbil Arzneimittel Gmbh, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Box - Box

This product is an oral powder containing 1,000 mg per packet of Sephience (sepiapterin). The directions for use recommend taking it with food. Each packet is a unit dose. The manufacturer of this product is PTC Therapeutics, Inc.*
Inner Label0 - Inner Label0

This text appears to contain information about a medication named "Sephience" in oral powder form, with each packet containing 1,000 mg. The product is associated with a specific NDC number (52856-301-01) and comes in unit-dose packets meant to be taken with food. The packaging includes usage directions and guidelines for administration. The medication is manufactured for PTC Therapeutics, Inc. with details on lot and expiration dates provided on the packaging.*
Inner Label2 - Inner Label2

This is a description of a medication called Sephience, which is an oral powder containing sepiapterin. Each packet contains 250 mg of the powder and should be taken with food. The medication is manufactured for PTC Therapeutics, Inc. The National Drug Code (NDC) for this medication is 52856-201-01. The packet is a unit-dose packet with instructions enclosed for administration.*
Outer Label0 - Outer Label0

This is a document providing information regarding a pharmaceutical product called Sephience, which is an oral powder containing 1,000 mg per packet of sepiapterin. The directions specify to take it with food, and there are 30 unit-dose packets in each box. The product does not contain preservatives, and any unused portion should be discarded. The recommended dosage and storage instructions are provided. The text also includes details about PTC Therapeutics, the manufacturer of Sephience.*
Outer Label2 - Outer Label2

This is a description of a medication called Sephience, which is in the form of an oral powder. Each packet contains 250 mg of the medication. The directions advise taking it with food. It is recommended to store Sephience between 20°C to 25°C (68°F to 77°F). This medication is manufactured for PTC Therapeutics, Inc., located in Warren, NJ, USA. The packaging contains 30 unit-dose packets. Additionally, it is mentioned that Sephience contains no preservatives and any unused portion should be discarded. For more information about dosage, one should refer to the Prescribing Information enclosed.*
Figure 1 - sephience 02

This text provides information on Mean Blood Phe levels (in μmol/L) at different time points in a study involving a placebo-controlled trial. The data shows the levels at baseline, week 12, week 34, and week 56 for both the placebo and the "Sephience" treatment.*
250mg Dose Packet - sephience 03

This description is for a product called Sephience, an oral powder form of sepiapterin, with 250 mg per packet. Each box contains 30 individual packets of this product.*
1000mg Dose Packet - sephience 04

This is information about Sephience oral powder containing 1,000 mg per packet (sepiapterin). It comes in a box of 30 packets, each of which is 1,000 mg. Sephience may be used for various purposes, but detailed usage instructions are not provided in the text.*
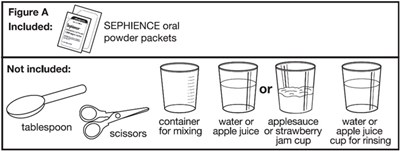
Figure A - sephience 05
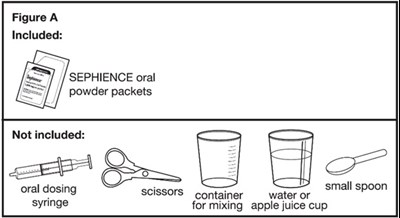
This is a list of items included and not included in a product package. The package includes SEPHIENCE oral powder packets, but does not include an oral dosing syringe or scissors. It also mentions a container for mixing water or apple juice.*
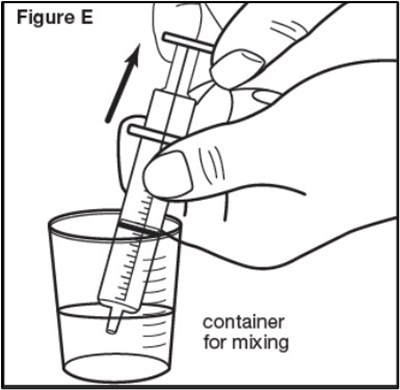
Figure B - sephience 06
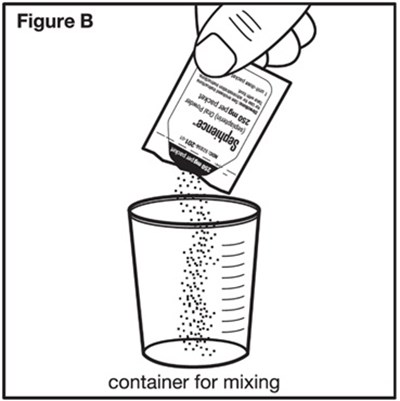
This text mentions a "Figure B" and a container used for mixing. It suggests that there may be an illustration or diagram showing a container designed for mixing substances or ingredients.*
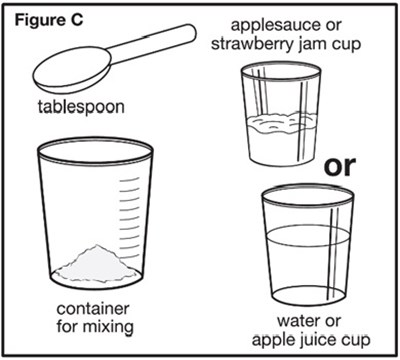
Figure C - sephience 07
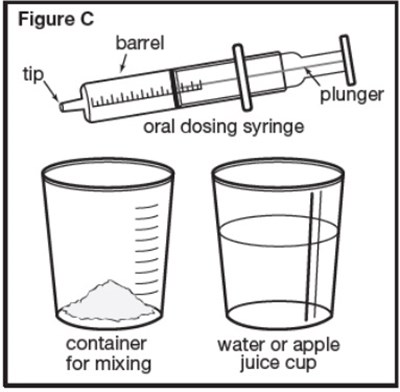
This text is a list of items including a barrel, plunger, oral dosing syringe, container for water or apple juice, and a mixing cup. These items appear to be related to a specific product or kit, possibly for medical or culinary purposes.*
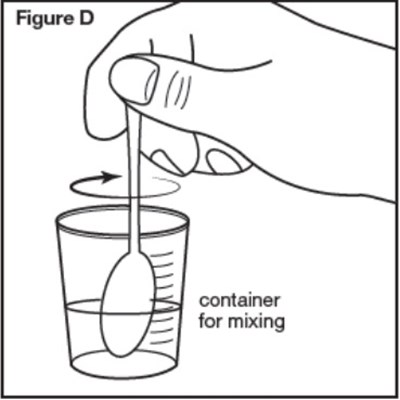
Figure D - sephience 08
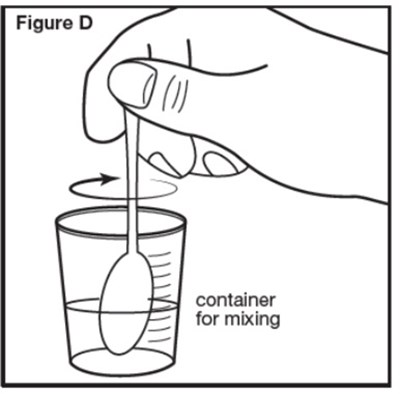
A container labeled "Figure D" is intended for mixing purposes, providing a convenient solution for blending different ingredients or substances together.*
Figure A1 - sephience 12
This is a list mentioning items included and not included with Figure A, which seems to be related to SEPHIENCE oral powder packets. The items mentioned include containers, water, applesauce, apple juice, strawberry jam, and a tablespoon. The purpose of these items may be for mixing or rinsing when using the oral powder packets.*
1000mg foil - sephience 14

This is a description of a product called "Sephience" (sepipten) oral powder that contains 250mg per packet.*
Figure C1 - sephience 16
This text provides instructions for making a fruit-based drink. It mentions using applesauce or strawberry jam in a cup, followed by adding a tablespoon of a liquid, likely water, and pouring apple juice into a container for mixing.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.