Product Images Fycompa
View Photos of Packaging, Labels & Appearance
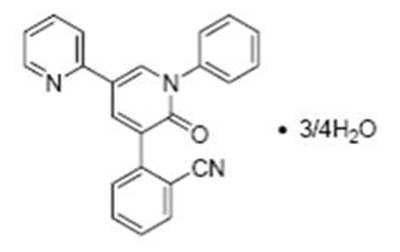
- chemical structure - fycompa 01
- Figure 1. Median Percent Reduction in Seizure Frequency per 28 Days from Baseline to Treatment Period - fycompa 02
- Figure 2. Proportion of Patients Exhibiting Different Percent Reductions During the Maintenance Phase Over Baseline Across All Three Trials. - fycompa 03
- Figure 3. Proportion of Patients Exhibiting Different Percent Reductions During the Maintenance Phase Over Baseline in Primary Generalized Tonic-Clonic Seizure Frequency. - fycompa 04
- Figure A - fycompa 05
- Figure B - fycompa 06
- Figure C - fycompa 07
- Figure D - fycompa 08
- Figure E - fycompa 09
- Figure F - fycompa 0a
- Figure G - fycompa 0b
- Figure H - fycompa 0c
- Figure I - fycompa 0d
- Figure J - fycompa 0e
- Figure K - fycompa 0f
- NDC 62856-272-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII2 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 10
- NDC 62856-274-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII4 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 11
- NDC 62856-276-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII6 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 12
- NDC 62856-278-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII8 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient - fycompa 13
- NDC 62856-280-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII10 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 14
- NDC 62856-282-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII12 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 15
- NDC 62856-290-380.5 mg/mLRx onlyFycompa®(perampanel)ORAL SUSPENSIONCIII0.5 mg/mLATTENTION PHARMACIST:Dispense the enclosedMedication Guide and Instructions for Usewith each prescription. - fycompa 16
- fycompa 17
- fycompa 18
- fycompa 19
- fycompa 1a
- fycompa 1b
- fycompa 1c
- fycompa 1d
- fycompa 1e
- fycompa 1f
- fycompa 20
- fycompa 21
- fycompa 22
- fycompa 23
- fycompa 24
- fycompa 25
- fycompa 26
- fycompa 27
- fycompa 28
- fycompa 29
- fycompa 2a
Product Label Images
The following 42 images provide visual information about the product associated with Fycompa NDC 62856-282 by Eisai Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1. Median Percent Reduction in Seizure Frequency per 28 Days from Baseline to Treatment Period - fycompa 02

This text describes the results of three different clinical studies (Study 1, Study 2, and Study 3) evaluating the effectiveness of a medication called FYCOMPA, given at doses of either 8mg, 12mg or 4mg per day. The studies compared the frequency of seizures in patients receiving FYCOMPA versus a placebo. The results show that in all three studies, the percentage reduction of seizures in patients taking FYCOMPA was statistically significant compared to those taking a placebo.*
Figure 2. Proportion of Patients Exhibiting Different Percent Reductions During the Maintenance Phase Over Baseline Across All Three Trials. - fycompa 03
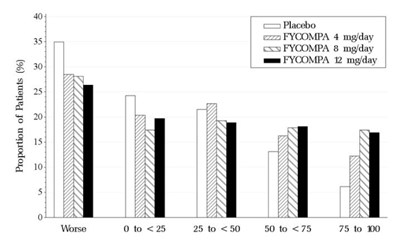
Figure 3. Proportion of Patients Exhibiting Different Percent Reductions During the Maintenance Phase Over Baseline in Primary Generalized Tonic-Clonic Seizure Frequency. - fycompa 04

This appears to be a chart showing the proportion of patients across different treatment groups for a certain medical condition. The treatment groups are "Placbo" (presumably placebo), and "FYCOMPA". The chart displays percentages of patients falling within different categories ranging from 0 to 75 (presumably a measure of severity), with 10% increments. Without additional context or labeling, the meaning of the patient percentages and severity categories cannot be determined.*
Figure D - fycompa 08
This is a simple description of an object consisting of an image labeled "Figure C" and a short text string "bottle adapter", suggesting that the image depicts a device used to adapt or connect different types of bottles.*
NDC 62856-272-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII2 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 10

NDC 62856-274-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII4 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 11

This is a medication named Fycompar, which is presented in tablet form of 2 mg. The tablets are packed in a bottle of 30 tablets, and it is only available through a prescription. The medication must be stored at a temperature between 15°C-30°C (69°F-86°F). The prescription information is available along with the medication. Additionally, there is a request to the pharmacist to provide the accompanying medication guide to each patient. The medication was manufactured by Eisai Ic.*
NDC 62856-276-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII6 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 12

This text describes the proper storage conditions (25°C-30°C) for Fycompa tablets manufactured by Esai Inc. and the dosage information is not available. It also indicates that the medication should be prescribed and dispensed by pharmacists along with the medication guide. The product is distributed in the US and comes in a pack of 30 tablets. The specific lot/expression and serialization area is also mentioned.*
NDC 62856-278-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII8 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient - fycompa 13

This is a label for Fycompa, a medication containing perampanel, in the form of tablets. The recommended storage temperature is 25°C (77°F) with permitted excursions between 15°C-30°C (s0°F-86°F). The dosage and usage instructions are not mentioned, but it suggests referring to the accompanying prescribing information. The label also includes the vendor's details and a batch number. Finally, it instructs the pharmacist to provide a medication guide to each patient upon dispensing.*
NDC 62856-280-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII10 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 14

Fycompar is a medication in tablet form with a concentration of 2 mg. The package contains a two week sample kit with fourteen 2 mg tablets. This medication requires a prescription and patients must receive the accompanying medication guide. The warning label on the package advises to keep it out of reach of children.*
NDC 62856-282-9090 tabletsRx onlyFycompa™(perampanel)tabletsCIII12 mgATTENTION PHARMACIST:Dispense the accompanyingMedication Guide to each patient. - fycompa 15

Fycompa is a medication available in tablet form for oral administration. This sample kit package contains fourteen 2mg tablets. The tablets should be pushed through the card from the inside before use. The medication should be stored at room temperature between 15°C-30°C (59°F-86°F). The recommended dosage for week 2 is one tablet once daily at bedtime. More details on dosage and usage are available with accompanying prescribing information. The Placeholder Dimensions are 3.8125 inches X 1.53125 inches.*
NDC 62856-290-380.5 mg/mLRx onlyFycompa®(perampanel)ORAL SUSPENSIONCIII0.5 mg/mLATTENTION PHARMACIST:Dispense the enclosedMedication Guide and Instructions for Usewith each prescription. - fycompa 16

This is a packaging of Fycompa tablets, which contain perampanel as the active ingredient. The tablets come in a 2mg dosage and the package contains a two-week supply with 14 tablets. There is a note for physicians to ensure their patients receive the accompanying medication guide before taking Fycompa.*
fycompa 17

Fycompa is a medication in the form of tablets containing 2mg of perampanel. The package includes fourteen tablets that need to be pushed through the card from inside. The tablets should be stored at a controlled room temperature of 25°C with permitted excursions to 15°C-30°C (59°F-86°F). The recommended dosage of Fycompa is one tablet orally, once daily at bedtime. Further prescribing information can be found in the accompanying package. The dimensions of the placeholder are 3.8125x1.53125 inches.*
fycompa 18

Store 30 tablets of Fycompa (perampanel) at 25°C (77°F), with excursions permitted to Rx only 15°C-30°C (59°F-86°F). The product has an NDC number of 62856-274-30. The prescribing information indicates that the medication guide should be dispensed to each patient.*
fycompa 19

This is a prescription drug called Fycompa (perampanel) manufactured in Japan and distributed by Eisai Inc. in Nutley, NJ. The drug comes in tablet form and should be stored at room temperature between 15°C-30°C (59°F-86°F). It is important for the pharmacist to dispense the accompanying medication guide to each patient. Additional information on dosage and use can be found in the prescribing information. The package contains 30 tablets and the lot/exp number is not available in the given text.*
fycompa 1a

Store at 25°C (77°F); excursions permitted to 15°C-30°C (sS°F-86°F). See USP Controlled Room Temperature. Dosage and use can be found in accompanying prescribing information. This is a medication prescribed by a doctor only and comes with a Medication Guide that should be given to the patient. The text includes a product name (Fycompa) and a lot number (NOC62856-274-90) with 90 tablets.*
fycompa 1b

This appears to be a medication box for Fycompa (perampanel) tablets, including a 2 Week Sample Kit and a Medication Guide. The instructions mention pushing a tablet through a card from the inside. No other information is available.*
fycompa 1c
This is a medication instruction for Fycompa tablets. The recommended dosage is to take one tablet orally at bedtime daily. The package contains 14 tablets of 4 mg each. The dosage and other related information are provided in the accompanying prescription information. The rest of the text is not readable or not available.*
fycompa 1d

This is a prescription medication called Fycompar, which is available in tablet form. It should be stored at room temperature, between 15-30°C (59°F- 86°F) and should be kept away from excursions. The dosage and usage information are available in the accompanying prescription information. This medication has been distributed by Eisai Inc., and the packaging also includes a medication guide to be given to each patient. Lot and expiration details are also mentioned in the text.*
fycompa 1e

This is a medication that needs to be stored at a temperature of 25°C (77°F) with permissible excursions between 15°C-30°C (59°F-86°F). It's essential to see the prescribing information for dosage and use. The medication is distributed by Eisai Inc. made in Japan, and it's accompanied by a Medication Guide that should be dispensed to every patient. The rest of the text contains information on LOT/EXP, the serialization area, and NDC 62856-276-30.*
fycompa 1f

Store the Fycompa (perampanel) tablets at a controlled room temperature between 15°C-30°C (5°F-86°F). The recommended dosage and usage of the medication is not available. The product comes in a 90-tablet pack with NDC62856-276-90 and is for prescription use only. Pharmacists are advised to dispense a medication guide to each patient.*
fycompa 20

Store the medication at room temperature between 15°C-30°C (59°F-86°F) in a controlled environment. This bottle contains 30 tablets of perampanel distributed by Eisai Inc. in Nutley, NJ. Please refer to the accompanying dosage and usage information. This medication requires a prescription and the pharmacist should dispense the accompanying medication guide to each patient.*
fycompa 22

This is a medication label for Fycompa tablets, with directions to store the medication at a temperature range of 15°C-30°C (59°F-86°F) and a warning to pharmacists to dispense the medication guide to each patient. The lot number and expiration date are not provided. The address of the manufacturer is given as Woodcliff Lake, NJ 07677.*
fycompa 23

This is the label of a medication called Fycompar, distributed by Eisai Inc. The medication is in tablet form and comes in a bottle with 30 tablets. The text provides information on the recommended storage temperature, dosage and use. The label also instructs pharmacists to dispense a medication guide to each patient. There is also a Lot/EXP Serialization area.*
fycompa 24

Store the medication at a temperature range of 15°C-30°C (59°F-86°F) with excursions permitted to R only, as indicated by NDC62856-280-30. This medicine is known as Fycompa (perampanel) and is distributed by Eisai Inc. Pharmacies are advised to dispense the accompanying medication guide to each patient.*
fycompa 25

This is a prescription drug packaging label for Fycompa (perampanel) tablets. It indicates that the tablets should be stored at a temperature of 25°C (77°F), with excursions permitted within a range of 15°C-30°C (S0°F-06°F), in accordance with USP Controlled D requirements. The medication is manufactured by Eisai and contains 90 tablets with the National Drug Code (NOC) 62856-260-90. The label also includes a warning for pharmacists to dispense the accompanying medication guide to each patient.*
fycompa 26

This text provides information about storing and dispensing NDC 62856-282-30 30 tablets of perampanel. The recommended storage temperature is between 15°C-30°C (59°F-86°F), with a permitted temperature excursion to Rx only 25°C (77°F). It also notes that an accompanying medication guide should be dispensed to each patient by the pharmacist. The product is distributed by Eisa Inc. and made in Italy.*
fycompa 27

This is a prescription drug called Fycompa (perampanel) and the package contains 30 tablets. It should be stored at room temperature (25°C or 77°F) and can be stored within a temperature range of 15°C-30°C (59°F-86°F). The accompanying prescribing information should be consulted before use. The drug is distributed by Eisai Inc. The package includes a Medication Guide that should be provided to each patient by the pharmacist. There is a lot/exp serialization area on the package.*
fycompa 28

This appears to be the label or packaging of a medication named "camsemine" in tablet form. It provides information about the temperature range suitable for storing the medication and refers to additional prescribing information that should be consulted for dosage and use details. The manufacturer or distributor of the medication is identified as "Eal e WoadkifLake" with a mailing address in K 07677.*
fycompa 29

Fycompa is an oral suspension medication with concentration of 0.5mg/mL. The medication should be discarded if unused after 90 days of first opening. It should be stored under 30°C (86°F) and should NOT be frozen.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.