Product Images Losartan Potassium
View Photos of Packaging, Labels & Appearance
- Structural Formula - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 01
- Figure 1 - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 02
- Figure 2 - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 03
- Figure 3 - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 04
- losartan - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 05
- 63187-830-90 - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 07
Product Label Images
The following 6 images provide visual information about the product associated with Losartan Potassium NDC 63187-830 by Proficient Rx Lp, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1 - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 02
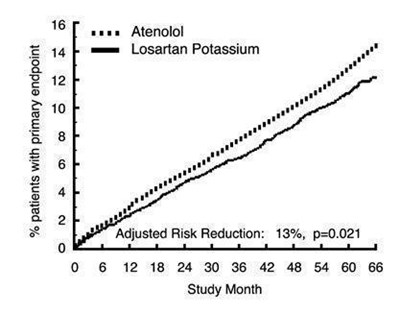
This text describes results from a study that compared the use of Atenolol and Osartan Potassium in patients. The study tracked patients for 66 months (5.5 years) and measured the percentage of patients who had the primary endpoint (not specified in this text). The adjusted risk reduction was found to be 13%, with a p-value of 0.021. There is a timeline of study months from 0 to 66.*
Figure 2 - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 03
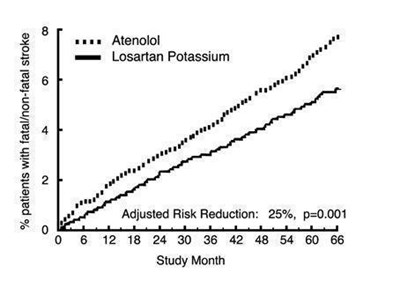
The text is reporting the percentage of patients with fatal/non-fatal stroke for two treatments, Atenolol and Losartan Potassium, along with an adjusted risk reduction of 25% with a p-value of 0.001. It also includes a chart with study months ranging from 0 to 66.*
63187-830-90 - 8ca0c9d3 3a50 4efe 92df 30a0c50d48a7 07

This is a description of a relabeled Losartan Potassium medication by Proficient Rx LP. The medication contains 50mg of Losartan Potassium in each of the 90 white to off-white, round and film-coated tablets, which have "S" and "Losartan Potassium 50mg 112" engraved on one side and a score line between 11 and 2 on the other side. It is a prescription-only medication and should be stored between 20°-25°C (68°-77°F) and kept out of reach of children. The originating manufacturer of the medication is Zhejiang Huahai Pharmaceutical Co., Ltd. in China. The lot number is 00000 and the expiration date is 00/00100.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.


