Product Images Otulfi
View Photos of Packaging, Labels & Appearance
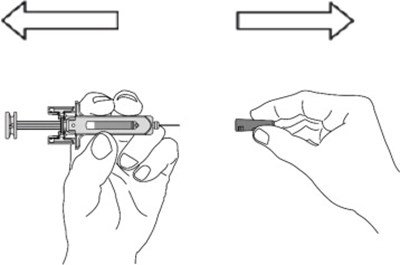
- Figure - otu00 0001 01
- Figure - otu00 0001 02
- Figure - otu00 0001 03
- Figure 1 - otu00 0001 04
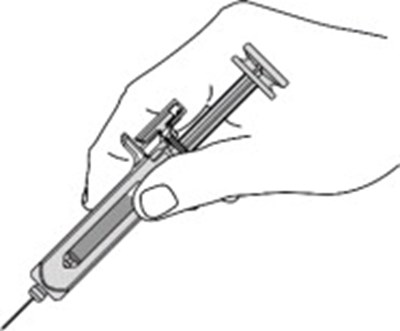
- Figure - otu00 0001 05
- Figure - otu00 0001 06
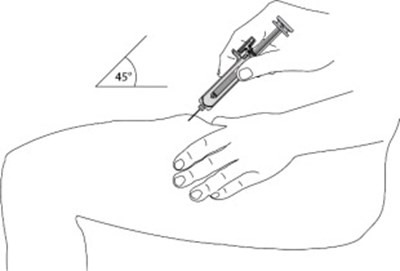
- Figure - otu00 0001 07
- Figure - otu00 0001 08
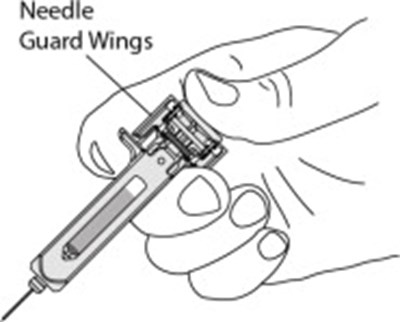
- Figure - otu00 0001 09
- Figure - otu00 0001 10
- Figure - otu00 0001 11
- Figure - otu00 0001 12
- Principal Display Panel – 45 mg Carton Label - otu00 0001 13
- Principal Display Panel – 45 mg Syringe Label - otu00 0001 14
- Principal Display Panel – 90 mg Carton Label - otu00 0001 15
- Principal Display Panel – 90 mg syringe Label - otu00 0001 16
- Principal Display Panel – 130 mg Carton Label - otu00 0001 17
- Principal Display Panel – 130 mg Vial Label - otu00 0001 18
Product Label Images
The following 18 images provide visual information about the product associated with Otulfi NDC 65219-828 by Fresenius Kabi Usa, Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
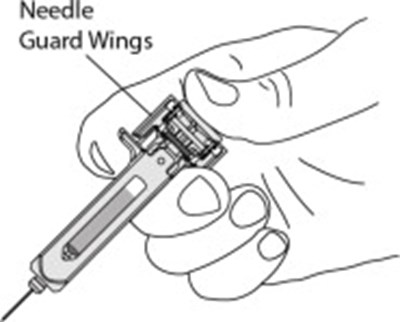
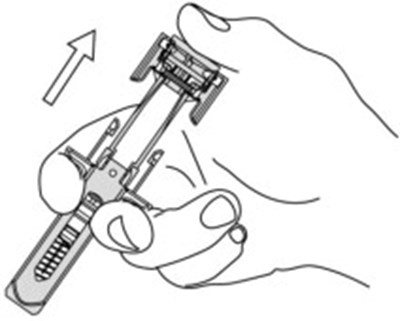
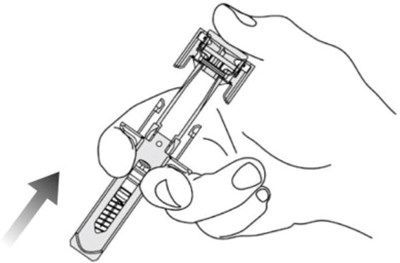
Figure - otu00 0001 06

This is a part of a medical device associated with a plunger and a needle guard for safe administration. It also includes activation clips and head wings for efficient operation. The text seems to be related to a medical injection device.*
Principal Display Panel – 45 mg Carton Label - otu00 0001 13

This text contains information about ustekinumab-aauz, an injection for subcutaneous use, stored in a refrigerator between 36°F to 46°F. It comes in a single-dose prefilled syringe that should be discarded after use. The active ingredient is 45 mg/0.5 mL of ustekinumab-aauz. It also provides details on inactive ingredients and instructions on storage and handling. For dosage information, please refer to the prescribing information.*
Principal Display Panel – 45 mg Syringe Label - otu00 0001 14

This is a single-dose prefilled syringe with the National Drug Code (NDC) 65219-824-01. It is advised to discard any unused portion and store the syringe at 36°F to 46°F (2°C to 8°C), away from light, and without shaking. Freezing is not recommended. This syringe is for subcutaneous use. The syringe also includes information on lot number and expiry date.*
Principal Display Panel – 90 mg Carton Label - otu00 0001 15

This text is a detailed description of ustekinumab-aauz, an injection for subcutaneous use. It includes information on dosage, storage, manufacturer, and disposal of the single-dose prefilled syringe. The text also contains details about the active and inactive ingredients in the medication, as well as guidance on reporting side effects and contacting a doctor for medical advice.*
Principal Display Panel – 90 mg syringe Label - otu00 0001 16

Single dose prefilled syringe with NDC 65219-826-26 is designed for subcutaneous use. The syringe should be stored at a cold temperature range of 36°F to 46°F to maintain its integrity. Users are advised to protect the injection from light and avoid shaking the syringe before use. The lot number and expiration date are mentioned on the packaging for reference.*
Principal Display Panel – 130 mg Carton Label - otu00 0001 17
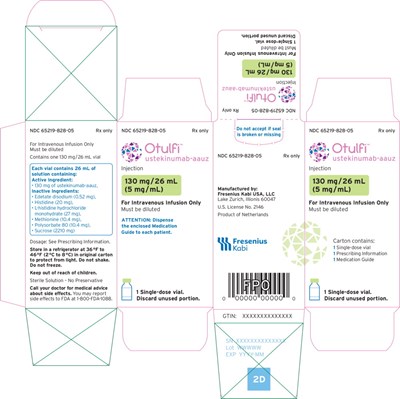
This is the detailed information about a medication with the NDC 65219-828-05. It is an intravenous infusion solution containing 130 mg of ustekinumab-aauz per 26 mL vial. The solution must be diluted before use and stored in a refrigerator at 36°F to 46°F. The medication includes both active and inactive ingredients. It is a sterile solution without preservatives. Patients should consult their doctor for medical advice and report any side effects to the FDA. The medication guide should be dispensed to each patient, and any unused portion should be discarded.*
Principal Display Panel – 130 mg Vial Label - otu00 0001 18
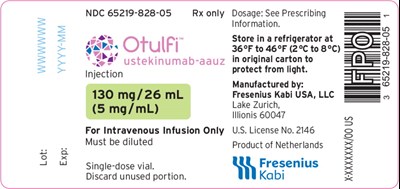
This is a product label for a medical injection, specifically a single-dose vial of 130 mg/26 mL solution for intravenous infusion only. The medication should be stored in a refrigerator at 36°F to 46°F (2°C to 8°C), in the original carton, and protected from light. It is manufactured by Fresenius Kabi USA, LLC and is a product of the Netherlands. The dosage information should be obtained from the prescribing information. Unused portions of the medication should be discarded.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.