FDA Label for Dawnmist Antiperspirant
View Indications, Usage & Precautions
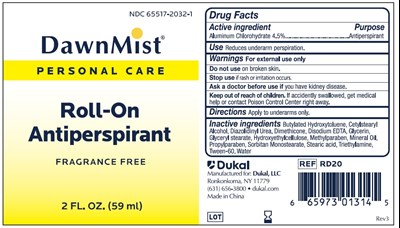
Dawnmist Antiperspirant Product Label
The following document was submitted to the FDA by the labeler of this product Dukal Llc. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Active Ingredient
Aluminum Chlorohydrate 4.5%
Purpose
Antiperspirant
Use
Reduces underarm perspiration
Warnings
For external use only.
Do Not Use
Do not use on broken skin
Ask A Doctor Before Use
Ask a doctor before use if you have kidney disease.
Keep Out Of Reach Of Children
Keep out of reach of children. If accidently swallowed, get medical help or contact Poison Control Center right away.
Directions
Apply to underarms only.
Inactive Ingredients
Butylated Hydroxytoluene, Cetylstearyl Alcohol, Diazolidinyl Urea, Dimethicone, Disodium EDTA, Glycerin, Glyceryl stearate, Hydroxyethylcellulose, Methylparaben, Mineral Oil, Propylparaben, Sorbitan Monostearate, Stearic acid, Triethylamine, Tween-60, Water
Principal Display Panel
NDC 65517-2032-1
DawnMist
Personal Care
Roll-On
Antiperspirant
Fragrance Free
2FL.OZ. (59ml)
* Please review the disclaimer below.