Product Images Ayuna
View Photos of Packaging, Labels & Appearance
- CIRCULATORY DISEASE MORTALITY RATES PER 100,000 WOMAN YEARS BY AGE, SMOKING STATUS AND ORAL CONTRACEPTIVE USE - levo ethi fig1
- Figure - levo ethi fig2
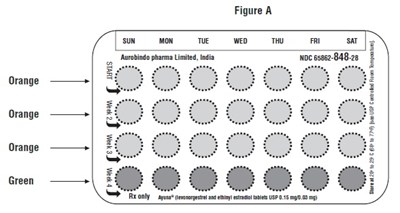
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.15 mg/0.03 mg (28 Tablets Pouch) - levo ethi fig3
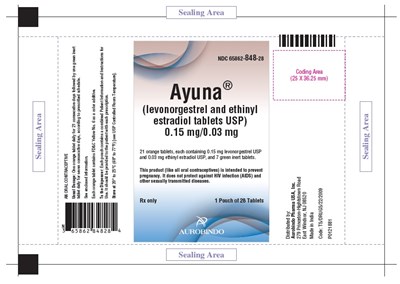
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.15 mg/0.03 mg (168 Tablets Carton) - levo ethi fig4
- levo ethi fig5
- levo ethi fig6
- levo ethi str1
- levo ethi str2
Product Label Images
The following 8 images provide visual information about the product associated with Ayuna NDC 65862-848 by Aurobindo Pharma Limited, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
CIRCULATORY DISEASE MORTALITY RATES PER 100,000 WOMAN YEARS BY AGE, SMOKING STATUS AND ORAL CONTRACEPTIVE USE - levo ethi fig1
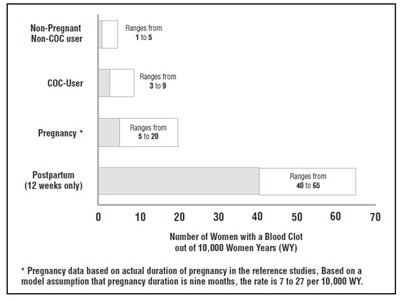
This is a table showing the number of women with a blood clot per 10,000 women years for different ranges of Non-Pregnant, Non-COC user, COC-User, and Postpartum women. There is also a note about the pregnancy range being based on actual pregnancy duration and that the rate of blood clots is between 7 to 27 per 10,000 WY.*
Figure - levo ethi fig2
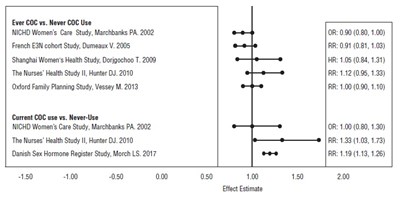
This text provides information regarding the use of COC (combined oral contraceptive) and its effects on women's health. The text presents a range of studies and their results in the form of odds ratios (OR), relative risks (RR), and hazard ratios (HR). The studies compare the use of COC vs. never using COC or current use vs. never use. The data suggests that there is a small association between ever using COC and a slight decrease in risk for certain health outcomes. However, the relationship between current use of COC and its impact on women's health is less clear. The text might be useful for medical professionals or researchers studying the effects of COC.*
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.15 mg/0.03 mg (28 Tablets Pouch) - levo ethi fig3
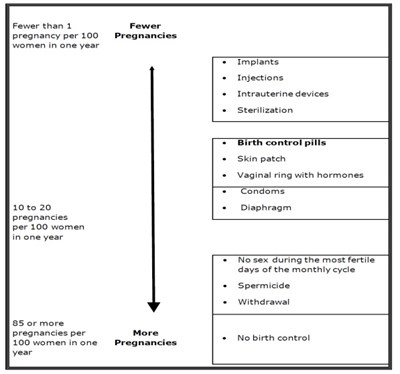
This document appears to be a chart that lists various forms of birth control and their effectiveness rates based on the number of pregnancies per 100 women in one year. It shows that some methods such as sterilization, intrauterine devices, and implants have a low rate of pregnancy, while others such as condoms and withdrawal have a higher rate. It also lists methods like birth control pills, skin patches, and vaginal rings as options. Lastly, it suggests the possibility of abstaining from sex during fertile times in the monthly cycle as a form of birth control.*
levo ethi fig6
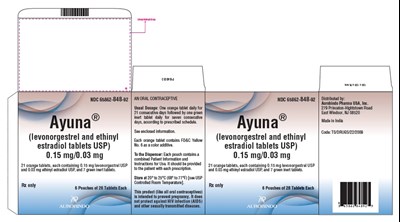
This is a description of an oral contraceptive product marketed under the brand name "ORANGEB." This contraceptive consists of tablets containing levonorgestrel and ethinyl estradiol, two hormones that prevent pregnancy by inhibiting ovulation. The recommended dosage is one tablet daily for 21 days, followed by a period of seven days without the medication. The tablets are available in two different strengths, 0.15 mg/0.03 mg and 0.5 mg, and are packaged in pouches containing 21 tablets each. The product should be stored at a temperature of 20-25°C.*
levo ethi str1
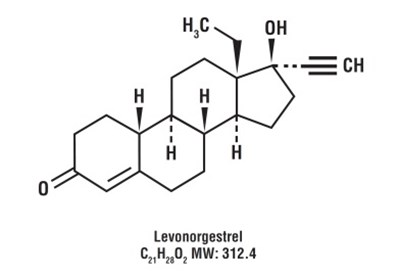
This is a chemical formula and abbreviation for Levonorgestrel. It does not provide any further useful description.*
levo ethi str2
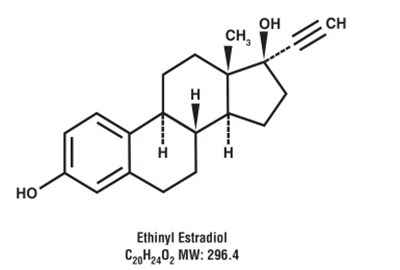
Ethinyl estradiol is a type of estrogen used in different forms of hormonal birth control. Its molecular weight is 296.4 g/mol.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.