Product Images Fondaparinux Sodium
View Photos of Packaging, Labels & Appearance
- Figure 1 and 2 - image 01
- Figure 3 and 4 - image 02
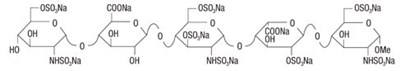
- Fondaparinux Sodium Structural Formula - image 03
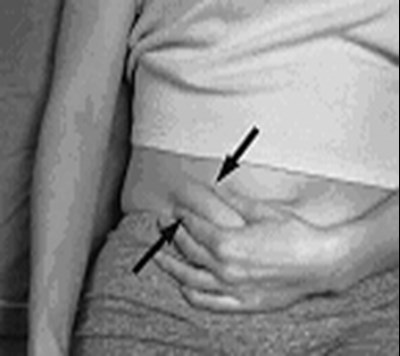
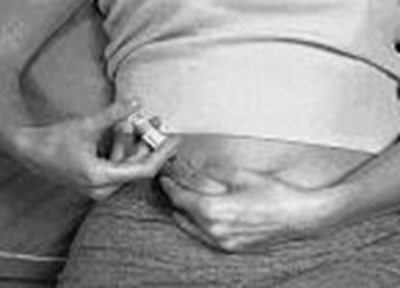
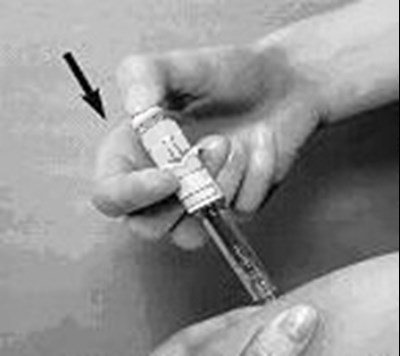
- arixtra-syringe-1 - image 04
- arixtra-syringe-2 - image 05
- arixtra-syringe-3 - image 06
- arixtra-figure-a - image 07
- arixtra-figure-b - image 08
- arixtra-figure-c - image 09
- arixtra-figure-d - image 10
- arixtra-figure-e - image 11
- arixtra-figure-f - image 12
- Fondaparinux Sodium Injection, USP for subcutaneous injection 5 mg/0.4 mL Carton Label - image 13
- Fondaparinux Sodium Injection, USP for subcutaneous injection 7.5 mg/0.6 mL Carton Label - image 14
- Fondaparinux Sodium Injection, USP for subcutaneous injection 10 mg/0.8 mL Carton Label - image 15
- image 16
Product Label Images
The following 16 images provide visual information about the product associated with Fondaparinux Sodium NDC 67457-582 by Mylan Institutional Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Fondaparinux Sodium Injection, USP for subcutaneous injection 5 mg/0.4 mL Carton Label - image 13

This is a description of a pharmaceutical product known as Fondaparinux Sodium Injection, USP used for Subcutaneous Injection. The product comes in a pack containing 10 single-dose, prefilled syringes for Rxonly which are affixed with an automatic needle protection system. The pharmacist is advised to dispense the accompanying patient information leaflot to each patient.*
Fondaparinux Sodium Injection, USP for subcutaneous injection 10 mg/0.8 mL Carton Label - image 15

This is a description of a drug called Fondaparinux Sodium Injection, USP. The drug comes in pre-filled syringes containing 75mg/0.6ml of the drug and is for subcutaneous injection only. The box contains ten single-dose syringes and is affixed with an automatic needle protection system. Additionally, the pharmacist is directed to dispense the patient information leaflet for each patient. The NDC number for the drug is 67457-584-06.*
image 16

Fondaparinux Sodium Injection is a medicine that is administered via subcutaneous injection. The medication is packaged in 10 pre-filled single-dose syringes, each with an automatic needle protection system. The package comes with a patient information leaflet, which should be dispensed to each patient. The rest of the text appears to be illegible.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.