FDA Label for Ampicillin And Sulbactam
View Indications, Usage & Precautions
- DESCRIPTION
- OTHER
- MICROBIOLOGY
- SUSCEPTIBILITY TESTING
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PREGNANCY
- ADVERSE REACTIONS
- DIRECTIONS FOR USE
- PREPARATION FOR INTRAVENOUS USE
- PREPARATION FOR INTRAMUSCULAR INJECTION
- HOW SUPPLIED
- REFERENCES
- STORAGE
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 1.5G VIAL LABEL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 1.5G CARTON LABEL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 3G VIAL LABEL
- PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 3G CARTON LABEL
Ampicillin And Sulbactam Product Label
The following document was submitted to the FDA by the labeler of this product Methapharm, Inc.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Description
Ampicillin and Sulbactam for Injection, USP is an injectable antibacterial combination consisting of the semisynthetic antibacterial ampicillin sodium and the beta-lactamase inhibitor sulbactam sodium for intravenous and intramuscular administration.
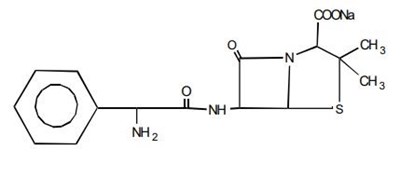
Ampicillin sodium is derived from the penicillin nucleus, 6-aminopenicillanic acid. Chemically, it is monosodium (2S, 5R, 6R)-6-[(R)-2-amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylate and has a molecular weight of 371.39. Its chemical formula is C16H18N3NaO4S. The structural formula is:
Sulbactam sodium is a derivative of the basic penicillin nucleus. Chemically, sulbactam sodium is sodium penicillinate sulfone; sodium (2S, 5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylate 4,4-dioxide. Its chemical formula is C8H10NNaO5S with a molecular weight of 255.22.
The structural formula is
Ampicillin and Sulbactam for Injection, USP, ampicillin sodium/sulbactam sodium parenteral combination, is available as a white to off-white dry powder for reconstitution. Ampicillin and Sulbactam for Injection, USP dry powder is freely soluble in aqueous diluents to yield pale yellow to yellow solutions containing ampicillin sodium and sulbactam sodium equivalent to 250 mg ampicillin per mL and 125 mg sulbactam per mL. The pH of the solutions is between 8.0 and 10.0.
Dilute solutions (up to 30 mg ampicillin and 15 mg sulbactam per mL) are essentially colorless to pale yellow. The pH of dilute solutions remains the same.
1.5 g of Ampicillin and Sulbactam for Injection, USP (1 g ampicillin as the sodium salt plus 0.5 g sulbactam as the sodium salt) parenteral contains approximately 115 mg (5 mEq) of sodium.
3 g of Ampicillin and Sulbactam for Injection, USP (2 g ampicillin as the sodium salt plus 1 g sulbactam as the sodium salt) parenteral contains approximately 230 mg (10 mEq) of sodium.
Other
General: Immediately after completion of a 15-minute intravenous infusion of ampicillin and sulbactam for injection, peak serum concentrations of ampicillin and sulbactam are attained. Ampicillin serum levels are similar to those produced by the administration of equivalent amounts of ampicillin alone. Peak ampicillin serum levels ranging from 109 to 150 mcg/mL are attained after administration of 2000 mg of ampicillin plus 1000 mg sulbactam and 40 to 71 mcg/mL after administration of 1000 mg ampicillin plus 500 mg sulbactam. The corresponding mean peak serum levels for sulbactam range from 48 to 88 mcg/mL and 21 to 40 mcg/mL, respectively. After an intramuscular injection of 1000 mg ampicillin plus 500 mg sulbactam, peak ampicillin serum levels ranging from 8 to 37 mcg/mL and peak sulbactam serum levels ranging from 6 to 24 mcg/mL are attained.
The mean serum half-life of both drugs is approximately 1 hour in healthy volunteers.
Approximately 75 to 85% of both ampicillin and sulbactam are excreted unchanged in the urine during the first 8 hours after administration of ampicillin and sulbactam for injection to individuals with normal renal function. Somewhat higher and more prolonged serum levels of ampicillin and sulbactam can be achieved with the concurrent administration of probenecid.
In patients with impaired renal function the elimination kinetics of ampicillin and sulbactam are similarly affected, hence the ratio of one to the other will remain constant whatever the renal function. The dose of ampicillin and sulbactam for injection in such patients should be administered less frequently in accordance with the usual practice for ampicillin (see DOSAGE and ADMINISTRATION section).
Ampicillin has been found to be approximately 28% reversibly bound to human serum protein and sulbactam approximately 38% reversibly bound.
The following average levels of ampicillin and sulbactam were measured in the tissues and fluids listed:
TABLE 1:
Concentration of Ampicillin and Sulbactam, in Various Body Tissues and Fluids
| Fluid or Tissue | Dose (grams) Ampicillin/Sulbactam | Concentration (mcg/mL or mcg/g) Ampicillin/Sulbactam |
| Peritoneal Fluid | 0.5/0.5 IV | 7/14 |
| Blister Fluid (Cantharides) | 0.5/0.5 IV | 8/20 |
| Tissue Fluid | 1/0.5 IV | 8/4 |
| Intestinal Mucosa | 0.5/0.5 IV | 11/18 |
| Appendix | 2/1 IV | 3/40 |
Penetration of both ampicillin and sulbactam into cerebrospinal fluid in the presence of inflamed meninges has been demonstrated after IV administration of ampicillin and sulbactam for injection.
The pharmacokinetics of ampicillin and sulbactam in pediatric patients receiving ampicillin and sulbactam for injection are similar to those observed in adults. Immediately after a 15-minute infusion of 50 to 75 mg ampicillin and sulbactam for injection/kg body weight, peak serum and plasma concentrations of 82 to 446 mcg ampicillin/mL and 44 to 203 mcg sulbactam/mL were obtained. Mean half-life values were approximately 1 hour.
Microbiology
Ampicillin is similar to benzyl penicillin in its bactericidal action against susceptible organisms during the stage of active multiplication. It acts through the inhibition of cell wall mucopeptide biosynthesis. Ampicillin has a broad spectrum of bactericidal activity against many gram-positive and gram-negative aerobic and anaerobic bacteria. (Ampicillin is, however, degraded by beta-lactamases and therefore the spectrum of activity does not normally include organisms which produce these enzymes).
A wide range of beta-lactamases found in microorganisms resistant to penicillins and cephalosporins have been shown in biochemical studies with cell free bacterial systems to be irreversibly inhibited by sulbactam. Although sulbactam alone possesses little useful antibacterial activity except against the Neisseriaceae, whole organism studies have shown that sulbactam restores ampicillin activity against beta-lactamase producing strains. In particular, sulbactam has good inhibitory activity against the clinically important plasmid mediated beta-lactamases most frequently responsible for transferred drug resistance. Sulbactam has no effect on the activity of ampicillin against ampicillin susceptible strains.
The presence of sulbactam in the ampicillin and sulbactam for injection formulation effectively extends the antibacterial spectrum of ampicillin to include many bacteria normally resistant to it and to other beta-lactam antibacterials. Thus, ampicillin and sulbactam for injection possesses the properties of a broad-spectrum antibacterial and a beta-lactamase inhibitor.
While in vitro studies have demonstrated the susceptibility of most strains of the following organisms, clinical efficacy for infections other than those included in the INDICATIONS AND USAGE section has not been documented.
Gram-Positive Bacteria: Staphylococcus aureus (beta-lactamase and non-beta-lactamase producing), Staphylococcus epidermidis (beta-lactamase and non-beta-lactamase producing), Staphylococcus saprophyticus (beta-lactamase and non-beta-lactamase producing), Streptococcus faecalis1 (Enterococcus), Streptococcus pneumoniae1 (formerly D. pneumoniae), Streptococcus pyogenes1, Streptococcus viridans1.
Gram-Negative Bacteria: Hemophilus influenzae (beta-lactamase and non-beta-lactamase producing), Moraxella(Branhamella)catarrhalis (beta-lactamase and non-beta-lactamase producing), Escherichia coli (beta-lactamase and non-beta-lactamase producing), Klebsiella species (all known strains are beta-lactamase producing), Proteus mirabilis (beta-lactamase and non-beta-lactamase producing), Proteus vulgaris, Providencia rettgeri, Providencia stuartii, Morganella morganii, and Neisseria gonorrhoeae (beta-lactamase and non-beta- lactamase producing).
Anaerobes:Clostridium species1, Peptococcus species1, Peptostreptococcus species, Bacteroides species, including B. fragilis.
________
1These are not beta-lactamase producing strains and, therefore, are susceptible to ampicillin alone.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Indications And Usage
Ampicillin and Sulbactam for Injection, USP is indicated for the treatment of infections due to susceptible strains of the designated microorganisms in the conditions listed below.
Skin and Skin Structure Infections caused by beta-lactamase producing strains of Staphylococcus aureus, Escherichia coli,2 Klebsiella spp.2 (including K. pneumoniae2), Proteus mirabilis,2 Bacteroides fragilis,2 Enterobacter spp.,2 and Acinetobacter calcoaceticus.2
NOTE: For information on use in pediatric patients see PRECAUTIONS – Pediatric Use and CLINICAL STUDIES sections.
Intra-Abdominal Infections caused by beta-lactamase producing strains of Escherichia coli, Klebsiella spp. (including K. pneumoniae2), Bacteroides spp. (including B. fragilis), and Enterobacter spp.2
Gynecological Infections caused by beta-lactamase producing strains of Escherichia coli,2 and Bacteroides spp.2 (including B. fragilis2).
__________
2Efficacy for this microorganism in this organ system was studied in fewer than 10 infections.
While Ampicillin and Sulbactam for Injection, USP is indicated only for the conditions listed above, infections caused by ampicillin-susceptible organisms are also amenable to treatment with Ampicillin and Sulbactam for Injection, USP due to its ampicillin content. Therefore, mixed infections caused by ampicillin-susceptible organisms and beta-lactamase producing organisms susceptible to Ampicillin and Sulbactam for Injection, USP should not require the addition of another antibacterial.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify the organisms causing infection and to determine their susceptibility to Ampicillin and Sulbactam for Injection, USP.
Therapy may be instituted prior to obtaining the results from bacteriological and susceptibility studies, when there is reason to believe the infection may involve any of the beta-lactamase producing organisms listed above in the indicated organ systems. Once the results are known, therapy should be adjusted if appropriate.
To reduce the development of drug-resistant bacteria and maintain effectiveness of Ampicillin and Sulbactam for Injection, USP and other antibacterial drugs, Ampicillin and Sulbactam for Injection, USP should be used only to treat infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Contraindications
The use of Ampicillin and Sulbactam for Injection USP is contraindicated in individuals with a history of hypersensitivity reactions (e.g. anaphylaxis or Steven-Johnson syndrome) to ampicillin, sulbactam or to other beta-lactam antibacterial drugs (e.g. penicillins and cephalosporins).
Ampicillin and sulbactam for injection is contraindicated in patients with a previous history of cholestatic jaundice/hepatic dysfunction associated with ampicillin and sulbactam for injection.
Warnings
Hypersensitivity
Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy. These reactions are more apt to occur in individuals with a history of penicillin hypersensitivity and/or hypersensitivity reactions to multiple allergens. There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins. Before therapy with a penicillin, careful inquiry should be made concerning previous hypersensitivity reactions to penicillins, cephalosporins, and other allergens. If an allergic reaction occurs, ampicillin and sulbactam for injection should be discontinued and the appropriate therapy instituted.
Hepatotoxicity
Hepatic dysfunction, including hepatitis and cholestatic jaundice has been associated with the use of Ampicillin and Sulbactam for injection. Hepatic toxicity is usually reversible; however, deaths have been reported. Hepatic function should be monitored at regular intervals in patients with hepatic impairment.
Severe Cutaneous Adverse Reactions
Ampicillin and sulbactam for injection may cause severe skin reactions, such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), dermatitis exfoliative, erythema multiforme, and Acute generalized exanthematous pustulosis (AGEP). If patients develop a skin rash they should be monitored closely and ampicillin and sulbactam for injection discontinued if lesions progress (see CONTRAINDICATIONS and ADVERSE REACTIONS sections).
Clostridium difficile-Associated Diarrhea: Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ampicillin and sulbactam for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Precautions
General: A high percentage of patients with mononucleosis who receive ampicillin develop a skin rash. Thus, ampicillin class antibacterials should not be administered to patients with mononucleosis. In patients treated with ampicillin and sulbactam for injection the possibility of superinfections with mycotic or bacterial pathogens should be kept in mind during therapy. If superinfections occur (usually involving Pseudomonas or Candida), the drug should be discontinued and/or appropriate therapy instituted.
Prescribing ampicillin and sulbactam for injection in the absence of proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Pregnancy
Reproduction studies have been performed in mice, rats, and rabbits at doses up to ten (10) times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to ampicillin and sulbactam for injection. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. (See – PRECAUTIONS - Drug/Laboratory Test Interactions section.)
Adverse Reactions
Adult Patients: Ampicillin and sulbactam for injection is generally well tolerated. The following adverse reactions have been reported in clinical trials.
Local Adverse Reactions
Pain at IM injection site – 16%
Pain at IV injection site – 3%
Thrombophlebitis – 3%
Phlebitis – 1.2%
Directions For Use
General Dissolution Procedures: Ampicillin and Sulbactam for Injection sterile powder for intravenous and intramuscular use, may be reconstituted with any of the compatible diluents described in this insert. Solutions should be allowed to stand after dissolution to allow any foaming to dissipate in order to permit visual inspection for complete solubilization.
Preparation For Intravenous Use
1.5 grams and 3 grams vials may be reconstituted with Sterile Water for Injection to yield solutions containing 375 mg ampicillin and sulbactam for injection per mL (250 mg ampicillin/125 mg sulbactam per mL).
An appropriate volume should then be immediately diluted with a suitable parenteral diluent to yield solutions containing 3 to 45 mg ampicillin and sulbactam for injection per mL (2 to 30 mg ampicillin/1 to 15 mg sulbactam/mL).
After the indicated time periods, any unused portions of solutions should be discarded.
TABLE 4:
| Diluent | Maximum Concentration (mg/mL) Ampicillin/Sulbactam | Use Periods |
| Sterile Water for Injection | 45 (30/15) 45 (30/15) 30 (20/10) | 8 hrs @ 25°C 48 hrs @ 4°C 72 hrs @ 4°C |
| 0.9% Sodium Chloride Injection | 45 (30/15) 45 (30/15) 30 (20/10) | 8 hrs @ 25°C 48 hrs @ 4°C 72 hrs @ 4°C |
| 5% Dextrose Injection | 30 (20/10) 30 (20/10) 3 (2/1) | 2 hrs @ 25°C 4 hrs @ 4°C 4 hrs @ 25°C |
| Lactated Ringer's Injection | 45 (30/15) 45 (30/15) | 8 hrs @ 25°C 24 hrs @ 4°C |
| M/6 Sodium Lactate Injection | 45 (30/15) 45 (30/15) | 8 hrs @ 25°C 8 hrs @ 4°C |
| 5% Dextrose in 0.45% Saline | 3 (2/1) 15 (10/5) | 4 hrs @ 25°C 4 hrs @ 4°C |
| 10% Invert Sugar | 3 (2/1) 30 (20/10) | 4 hrs @ 25°C 3 hrs @ 4°C |
Preparation For Intramuscular Injection
1.5 g and 3 g Vials: Vials for intramuscular use may be reconstituted with Sterile Water for Injection USP, 0.5% Lidocaine Hydrochloride Injection USP or 2% Lidocaine Hydrochloride Injection USP. Consult the following table for recommended volumes to be added to obtain solutions containing 375 mg ampicillin and sulbactam for injection per mL (250 mg ampicillin/125 mg sulbactam per mL). Note: Use only freshly prepared solutions and administer within one hour after preparation.
TABLE 5:
| Ampicillin and Sulbactam for Injection Vial Size | Volume of Diluent to be Added | Withdrawal Volume* |
| 1.5 g | 3.2 mL | 4.0 mL |
| 3 g | 6.4 mL | 8.0 mL |
*There is sufficient excess present to allow withdrawal and administration of the stated volumes.
How Supplied
Ampicillin and Sulbactam for Injection, USP (ampicillin sodium/sulbactam sodium) is supplied as a sterile white to off-white dry powder in glass vials. The following packages are available:
Vials containing 1.5 g (NDC 67850-130-10) equivalent of Ampicillin and Sulbactam for Injection, USP (1 g ampicillin as the sodium salt plus 0.5 g sulbactam as the sodium salt). 10 vials in a carton.
Vials containing 3 g (NDC 67850-131-10) equivalent of Ampicillin and Sulbactam for Injection, USP (2 g ampicillin as the sodium salt plus 1 g sulbactam as the sodium salt). 10 vials in a carton.
References
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Eleventh Edition. CLSI document M02-A11. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fourth Informational Supplement. CLSI document M100-S24. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2014.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard – Ninth Edition. CLSI document M07-A9, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard – Eighth Edition. CLSI document M11-A8. Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
Storage
Prior to reconstitution store dry powder at 20° to 25° C (68° to 77° F). [See USP Controlled Room Temperature].
Package Label - Principal Display Panel - 1.5G Vial Label
NDC 67850-130-00
Ampicillin and
Sulbactam
for Injection, USP
1.5 grams* per vial
For intravenous or Intramuscular Use
Single-Dose Vial. Sterile.
*Equivalent to 1g ampicillin
as the sodium salt plus 0.5g
sulbactam as the sodium salt.
The sodium content is about
115 mg (5mEq). Rx only
Package Label - Principal Display Panel - 1.5G Carton Label
10 Single-Dose Vials NDC 67850-130-10
Ampicillin and Sulbactam
for Injection, USP
1.5 grams* per vial
For intravenous or Intramuscular Use
Single-Dose Vial. Sterile.
*Equivalent to 1g ampicillin as the sodium salt plus 0.5g sulbactam
as the sodium salt. The sodium content is about 115 mg (5mEq).
Rx only
Sterile
Package Label - Principal Display Panel - 3G Vial Label
NDC 67850-131-00
Ampicillin and
Sulbactam
for Injection, USP
3 grams* per vial
For intravenous or Intramuscular Use
Single-Dose Vial. Sterile.
*Equivalent to 2g ampicillin
as the sodium salt plus 1g
sulbactam as the sodium salt.
The sodium content is about
230 mg (10mEq). Rx only
Package Label - Principal Display Panel - 3G Carton Label
10 Single-Dose Vials NDC 67850-131-10
Ampicillin and Sulbactam
for Injection, USP
3 grams* per vial
For intravenous or Intramuscular Use
Single-Dose Vial. Sterile.
*Equivalent to 2g ampicillin as the sodium salt plus 1g sulbactam
as the sodium salt. The sodium content is about 230 mg (10 mEq).
Rx only
Sterile
* Please review the disclaimer below.