Product Images Clopidogrel
View Photos of Packaging, Labels & Appearance
Product Label Images
The following 11 images provide visual information about the product associated with Clopidogrel NDC 69367-200 by Westminster Pharmaceuticals, Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1 - clopidogrel 02

This is a list of different doses (in milligrams) of four medications used to treat acid reflux or ulcers: Dexlansoprazole (60mg), Lansoprazole (30mg), Pantoprazole (80mg), and Omeprazole (80mg). There is no context or further information provided.*
Figure 2 - clopidogrel 03
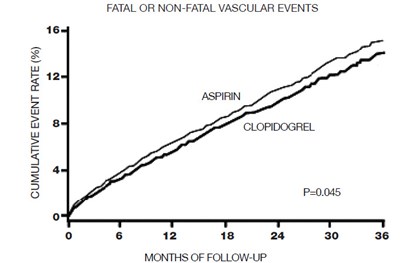
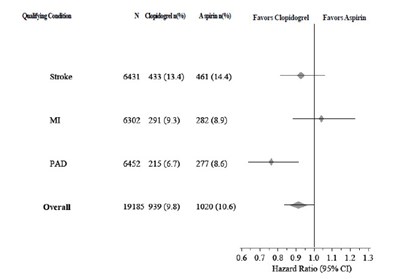
This text is a useful description of a comparative analysis of two drugs, placebo and clopidogrel, in the prevention of cardiovascular death, myocardial infarction, and stroke over a period of 12 months. It shows the cumulative event rate (%) of the three conditions and the months of follow-up. The results indicate that clopidogrel is more effective than placebo in preventing cardiovascular events. The study also notes that other standard therapies were used as appropriate.*
Figure 5 - clopidogrel 06
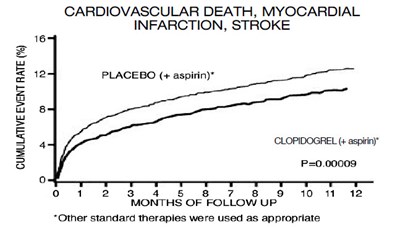
This is a summary of a clinical trial evaluating the efficacy of clopidogrel. The text compares the number of deaths that occurred before the first discharge in patients treated with placebo and clopidogrel. The placebo group had 1845 deaths (8.1%), while the clopidogrel group had 1726 deaths (7.5%), representing a 7% reduction in proportional risk (p=0.03). The graph shows the number of days since randomization (up to 28 days) on the X-axis, and the number of deaths on the Y-axis.*
Figure 6 - clopidogrel 07
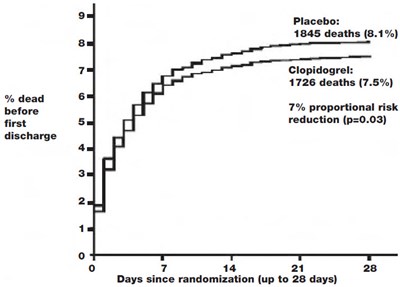
This text appears to be presenting data related to clinical trials for two treatments - J Placebo and Clopidogrel. The percentage of patients who experienced an event while taking J Placebo was 10.1%, while for Clopidogrel it was 9.2%. The data also suggests a reduction in re-infarction or stroke risk associated with Clopidogrel (p=0.002). The graph shows the number of days since randomization for patients, up to a maximum of 28 days.*
Figure 7 - clopidogrel 08
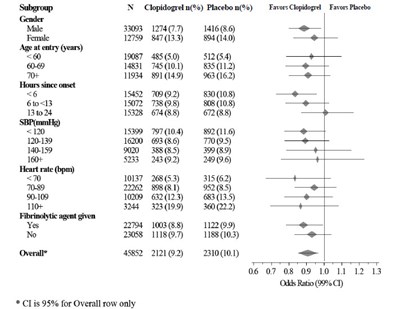
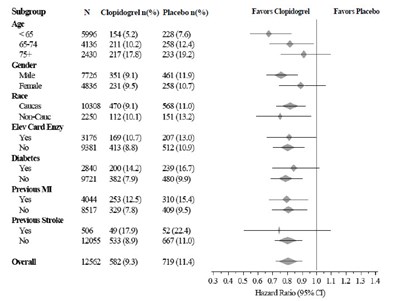
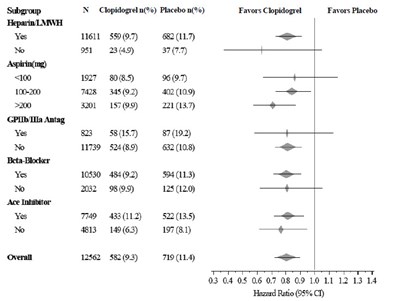
This is a formatted table of data with information on the percentage of patients taking Clopidogrel vs. Placebo in a clinical trial. The data is categorized based on subgroups including gender, age, hours since onset, SBP, heart rate, and whether or not a patient had a specific medical condition. The table also includes odds ratios with corresponding confidence intervals. There is not enough context to determine the purpose or results of the trial.*
clopidogrel 11
This is a description for Clopidogrel tablets. The text provides information on the usual adult dosage and storage conditions for this medication. It also indicates the manufacturer and distributor of the tablets. The recommended storage temperature is between 15°C to 30°C. The tablets contain 391 mg of clopidogrel butal and are equivalent to 300 mg of clopidogrel. The accompanying medication guide should be dispensed to each patient by the pharmacist.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.