FDA Label for Senna-s
View Indications, Usage & Precautions
Senna-s Product Label
The following document was submitted to the FDA by the labeler of this product Healthlife Of Usa Llc. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Other
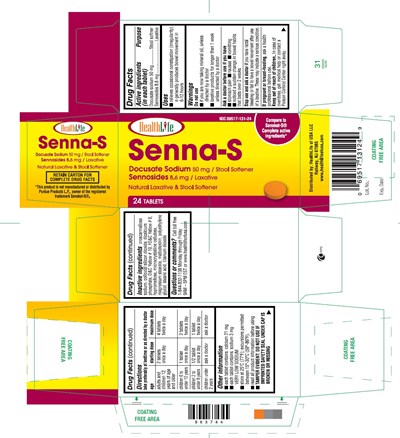
Drug Facts
Otc - Purpose
Active ingredients Purpose (in each tablet) Purpose
Docusate sodium 50 mg.......................................Stool softner
Sennosides 8.6 mg................................................Laxative
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6-12 hours
Do Not Use
- if you are now taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week unless directed by a doctor
Ask A Doctor Before Use If You Have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Stop Use And Ask A Doctor If
you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
Otc - Pregnancy Or Breast Feeding
If pregnant or breast-feeding, ask a health professional before use.
Otc - Keep Out Of Reach Of Children
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
take preferably at bedtime or as directed by a doctor
- adults and children 12 years of age or older - starting dosage: 2 tablets once a day, maximum dosage: 4 tablets twice a day
- children 6 to under 12 years - starting dosage: 1 tablet once a day, maximum dosage: 2 tablets twice a day
- children 2 to under 6 years - starting dosage: 1/2 tablet once a day, maximum dosage: 1 tablet twice a day
- children under 2 years - starting dosage: ask a doctor, maximum dosage: ask a doctor
Other Information
- each tablet contains: calcium 21 mg
- each tablet contains: sodium 3 mg VERY LOW SODIUM
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- read all product information before using
- TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Inactive Ingredients
croscarmellose sodium, colloidal silicon dioxide, dicalcium phosphate, D&C Yellow # 10, FD&C Yellow # 6, hypromellose, microcrystalline cellulose, magnesium stearate, maltodextrin, polyethylene glycol, stearic acid, titanium dioxide
Questions Or Comments?
Call toll free 1-844-832-1138 Monday through Friday 9AM – 5PM EST or www.healthlifeofusa.com
Principal Display Panel
Compare to the Active Ingredients in Senokot-S®
*This product is not manufactured or distributed by Purdue Products L.P., owner of the registered trademark Senokot-S®.
Senna and Docusate Sodium Tablets, 8.6 mg and 50 mg
* Please review the disclaimer below.