Product Images Ic-green
View Photos of Packaging, Labels & Appearance
Product Label Images
The following 4 images provide visual information about the product associated with Ic-green NDC 70100-825 by Renew Pharmaceuticals Limited, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Principal Display Panel - Vial - image 02

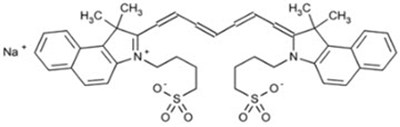
This is a product label for IC-GREEN® (indocyanine green for injection) containing 25 mg per vial. It is intended for single-patient-use for intravenous or interstitial administration. After reconstitution, it should be used within 6 hours. The product is sterile and can only be obtained with a prescription. The expiration date indicated on the label is 12/2024, and it includes a lot number for tracking purposes.*
Principal Display Panel - Sterile Water Vial - image 03

This is a 10mL single-dose drug intended for diluent use only. The product is sterile and nonpyrogenic, and does not contain any antimicrobial or other added substances. It is a prescription-only item with the NDC 0409-4887-17 and is manufactured by Hospira, Inc. in Lake Forest, IL.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.