Product Images Galantamine
View Photos of Packaging, Labels & Appearance
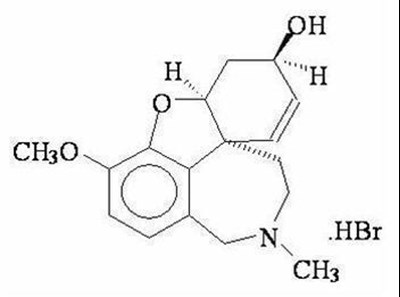
- Chemical Structure - galantamine 01
- image description - galantamine 02
- image description - galantamine 04
- image description - galantamine 05
- image description - galantamine 06
- image description - galantamine 07
- image description - galantamine 08
- image description - galantamine 09
- image description - galantamine 10
- image description - galantamine 11
- image description - galantamine 12
- galantamine 12mg 60 container label
- image description - galantamine 13
- galantamine 4mg 60 container label
- galantamine 8mg 60 container label
- galantamine03
Product Label Images
The following 16 images provide visual information about the product associated with Galantamine NDC 70436-005 by Slate Run Pharmaceuticals, Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
image description - galantamine 06
The text is only partially readable and does not provide enough information for a useful description.*
galantamine 12mg 60 container label

Each tablet of Galantamine hydrobromide contains 12 mg of galantamine, and it should be stored in a tight, light-resistant container at a temperature between 15°C to 30°C (59°F to 86°F). The dosage instructions should be read in the accompanying product literature. This medication is made by Yabao Pharmaceutical Co., Ltd. in Beijing, China and distributed by Slate Run Pharmaceuticals, LLC in Columbus, Ohio.*
galantamine 4mg 60 container label

This is a medication called Galantamine Tablets USP, produced by Yabao Pharmaceutical Co. in Beijing, China. Each tablet contains 4 mg of Galantamine hydrobromide, and it comes in a container that is defined in the USP. The dosage of the medication is available in the enclosed literature. The drug must be kept out of reach of children and stored in a tight and light-resistant container at 25°C (77°F). It can be kept at excursions permitted to 15°C to 30°C (59°F to 86°F). The distributor is N Slate Run Pharmaceuticals, LLC.*
galantamine 8mg 60 container label

This is a medication containing Galantamine hydrobromide in tablets form. Each tablet contains a USP equivalent of 8 mg Galantamine. The dosage should be as per the accompanying product literature. It should be stored in a tight, light-resistant container at 25°C (77°F) with permitted excursions from 15°C to 30°C (59°F to 86°F) [See USP o Tablets USP Controlled Room Temperature]. This medication is manufactured by Yabao Pharmaceutical Co., Ltd, Beijing, China 101111 and distributed by Slate Run Pharmaceuticals in Columbus, Ohio. The NDC number for this drug is 70436-005-06. It is advised to keep this and all drugs out of the reach of children.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.