Product Images Pregabalin
View Photos of Packaging, Labels & Appearance
- Pregabalin 75mg 70518 2525 00
- equation - pregabain gault equation
- fig-01 - pregabalin figure 01
- fig-02 - pregabalin figure 02
- fig-03 - pregabalin figure 03
- fig-04 - pregabalin figure 04
- fig-05 - pregabalin figure 05
- fig-06 - pregabalin figure 06
- fig-07 - pregabalin figure 07
- fig-09 - pregabalin figure 09
- fig-10 - pregabalin figure 10
- fig-11 - pregabalin figure 11
- fig-12 - pregabalin figure 12
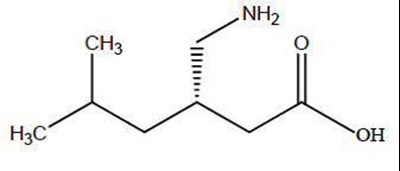
- str - pregabalin structure
Product Label Images
The following 14 images provide visual information about the product associated with Pregabalin NDC 70518-2525 by Remedyrepack Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Pregabalin 75mg 70518 2525 00

This is a description of a medication named Pregabalin. The medication comes in a bottle with a National Drug Code number of 70518-2525-00 and contains 90 capsules of 75 milligrams each. The medication was manufactured by Novadoz Pharmaceuticals LLC in New Jersey, US. The lot number and expiration date are not available. The source NDC number is 72205-0013-90. The medication should be kept out of reach of children and stored in a cool and dry place at a temperature between 20-26°C (63-77°F), with excursions between 15-30°C (59-86°F). It is for Rx only and has been repackaged by RemedyRepack Inc. in Indiana, PA. *
equation - pregabain gault equation
This is a formula for calculating creatinine clearance in patients. It takes into account the patient's age, weight, and serum creatinine levels. If the patient is female, the result is multiplied by 0.85.*
fig-02 - pregabalin figure 02
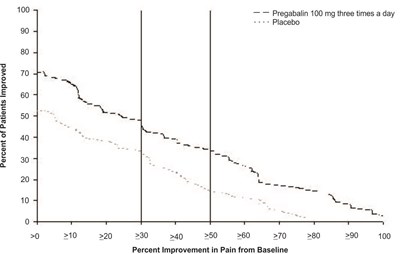
This is a graph showing the percentage of patients who improved after taking Pregabalin 100 mg three times a day compared to a placebo. The improvement in pain from baseline is also displayed in percentages ranging from 10 to 100.*
fig-04 - pregabalin figure 04
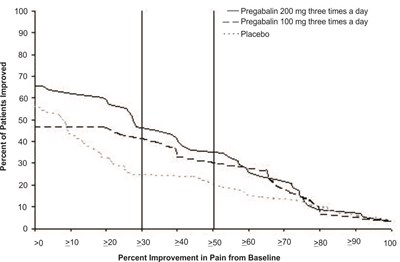
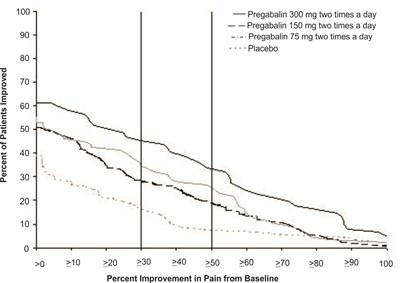
This appears to be a graph or chart showing the percentage of patients who improved based on different levels of pain relief from baseline. The x-axis measures different improvement levels (10%, 20%, 30%, etc.) while the y-axis measures the percentage of patients who reported improvement. Three treatments are being compared: Pregabalin 200 mg three times a day, Pregabalin 100 mg three times a day, and a placebo.*
fig-05 - pregabalin figure 05

The text shows a table that displays the percentage of patients who saw an improvement in pain from baseline when taking pregabalin at varying doses as compared to placebo. The table also includes the percent improvement in pain from baseline at different levels.*
fig-06 - pregabalin figure 06

The text appears to be a table showing responder rates (%) from a study or studies (E1 and E3) with different dosages (50mg/day, 150mg/day, 300mg/day, 600mg/day) of a drug compared to a placebo. The data is presented for different dosing frequencies (BID and TID). There is a statistically significant difference in response rates between the drug and placebo at certain dosages. There is one cell with the value of 14%, and another cell with the value of 15% that doesn't seem to correspond with any particular dosage or frequency. However, without additional context, it is difficult to determine the purpose or significance of this table.*
fig-07 - pregabalin figure 07
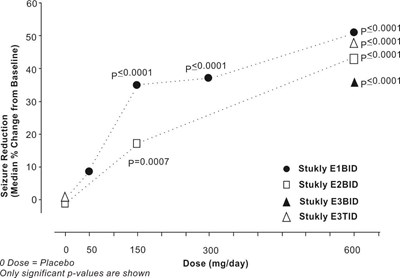
This is a chart displaying results of a study, with values and p-values for different doses. The doses are listed in mg/day, with a placebo listed at 0 mg/day. There are different groups indicated by names such as Stukly E1BID, etc. The chart shows values for each group at different doses, with significant p-values indicated.*
fig-09 - pregabalin figure 09
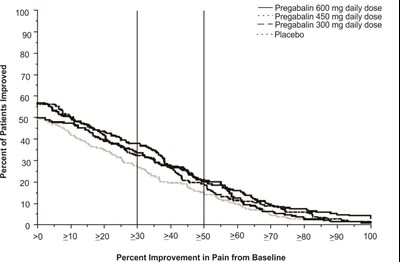
This appears to be a chart showing the percentage of patients who experienced improvements in pain from baseline after taking different doses of Pregabalin or placebo. The x-axis shows the percentage improvement in pain while the y-axis shows the percentage of patients who experienced that level of improvement. There are four lines on the chart, representing different daily doses of Pregabalin (600 mg, 450 mg, and 300 mg) and a placebo group. The chart shows that a higher percentage of patients experienced improvement in pain with increasing doses of Pregabalin compared to the placebo group.*
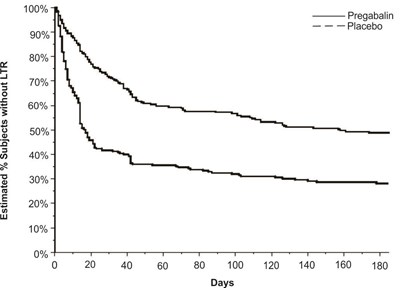
fig-11 - pregabalin figure 11

The text is a graph showing the percent of subjects who improved from baseline to week 12 with the drug Pregabalin compared to a placebo. The graph shows values at various intervals from 0 to 100.*
fig-12 - pregabalin figure 12
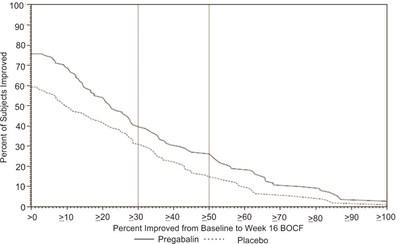
This table shows the percentage of subjects who showed improvement during a 16-week treatment period with either Pregabalin or Placebo, using BOCF B as the measure. The percentages range from 0 up to 100 with increments of 10, and the text indicates the number of subjects who demonstrated improvement at or above each increment.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.