Product Images Metoprolol Succinate
View Photos of Packaging, Labels & Appearance
Product Label Images
The following 3 images provide visual information about the product associated with Metoprolol Succinate NDC 70518-4198 by Remedyrepack Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Remedy_Label - Remedy Label

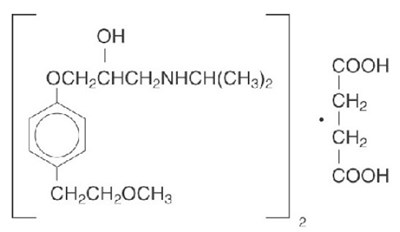
This is a 25 mg Metoprolol Succinate extended-release tablet prescription, with a quantity of 90 tablets. The manufacturer is Ascend Labs, LLC based in Montvale, NJ. The medication should be stored at 20-25°C (58-77°F) with excursions permitted to 15-30°C (59-86°F). For more information on usage, refer to the package insert. Keep out of reach of children.*
metoprolol-table1a - metoprolol table1a
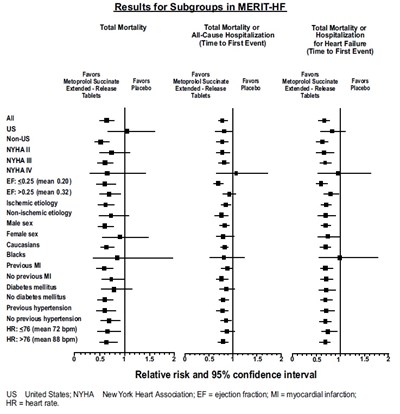
This is a report on the results of subgroups in the MERIT-HF study related to total mortality, all-cause hospitalization, and heart failure outcomes. The study compares the effects of Metoprolol Succinate Extended-Release tablets with a placebo on different subgroups including NYHA classification, ejection fraction, etiology of heart failure, sex, race, history of myocardial infarction, diabetes mellitus, hypertension, and heart rate. The study presents relative risk and 95% confidence intervals for each subgroup.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.