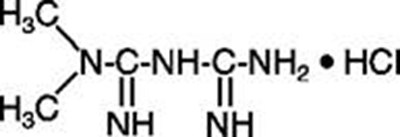Product Images Metformin Hydrochloride
View Photos of Packaging, Labels & Appearance
Product Label Images
The following 3 images provide visual information about the product associated with Metformin Hydrochloride NDC 70518-4369 by Remedyrepack Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
MM2 - Metformin HCl 1000mg 70518 4369 01

This is a description for Metformin HCI tablet with 1000 mg strength. The tablets are extended-release and should not be broken, crushed, or chewed. The medication is manufactured by Lupin Pharma and repackaged by RemedyRepack Inc. It should be stored at 20-25°C, with excursions permitted to 15-30°C. The lot number, expiration date, and other details are not available in the text.*
Remedy_Label - Remedy Label

This is information about Metformin HCI tablets, with a dosage of 1000 mg. The tablets are extended-release and should not be broken, crushed, or chewed. The manufacturer is Lupin Pharma, based in Baltimore, MD. The tablets should be stored at 20-25°C (68-77°F) and protected from excessive heat and humidity. The package contains 30 tablets, and they should be kept out of reach of children. The text also includes details about the expiration date, repackaging information, and contact details for RemedyRepack Inc.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.