Product Images Docetaxel Anhydrous
View Photos of Packaging, Labels & Appearance
- Principal Display Panel – Docetaxel Injection, USP 16 mL Carton - 160mg cart np
- Principal Display Panel – Docetaxel Injection, USP 16 mL Vial Label - 160mg vial np
- Principal Display Panel – Docetaxel Injection, USP 2 mL Carton - 20mg cart np
- Principal Display Panel – Docetaxel Injection, USP 2 mL Vial Label - 20mg vial np
- Principal Display Panel – Docetaxel Injection, USP 8 mL Carton - 80mg cart np
- Principal Display Panel – Docetaxel Injection, USP 8 mL Vial Label - 80mg vial np
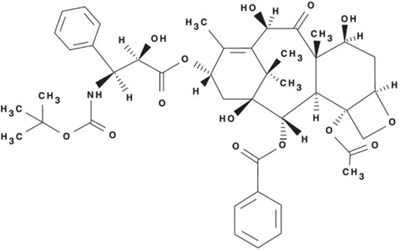
- Docetaxel Structural Formula - doc09 0000 01
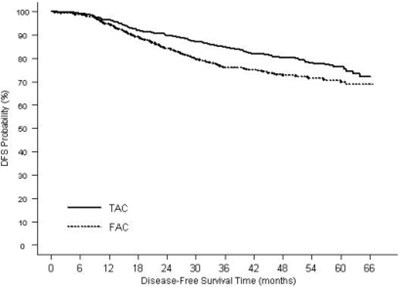
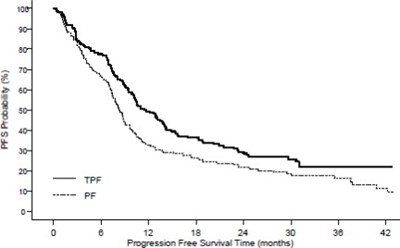
- Figure 1 - doc09 0000 02
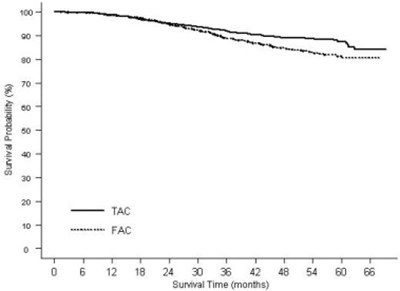
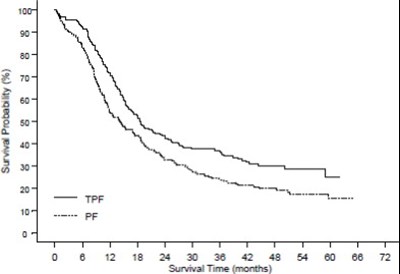
- Figure 2 - doc09 0000 03
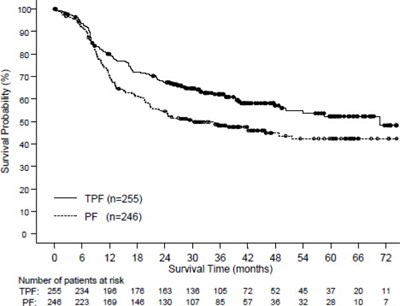
- Figure 3 - doc09 0000 04
- Figure 4 - doc09 0000 05
- Figure 5 - doc09 0000 06
- Figure 6 - doc09 0000 07
- Figure 7 - doc09 0000 08
- Figure 8 - doc09 0000 09
- Figure 9 - doc09 0000 10
- Figure 10 - doc09 0000 11
Product Label Images
The following 17 images provide visual information about the product associated with Docetaxel Anhydrous NDC 71288-150 by Meitheal Pharmaceuticals Inc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Principal Display Panel – Docetaxel Injection, USP 2 mL Carton - 20mg cart np
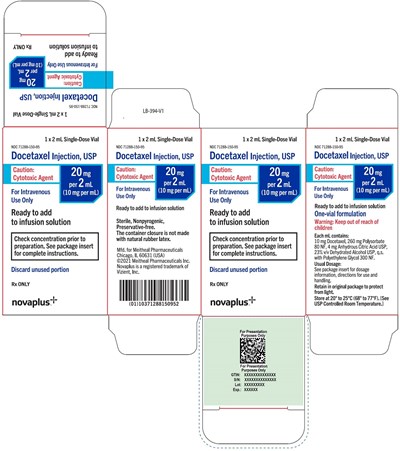
This document appears to be a pharmaceutical company's product labeling for their Docetaxel Injection, a cytotoxic agent that is administered intravenously as a single-dose vial. The labeling includes warnings and dosage instructions, and recommends keeping the container away from natural rubber latex and out of reach of children. It also summarizes information about storage conditions and tells pharmacists to discard any unused medication.*
Principal Display Panel – Docetaxel Injection, USP 2 mL Vial Label - 20mg vial np

This is a description for a medication called Docetaxel injection, used for intravenous use only as a cytotoxic agent. It comes in a 2 mL single-dose vial with 20 mg dosage. Any unused portion should be discarded. The medication is a one-vial formulation and is ready to add to the infusion solution. The package insert should be consulted for complete instructions. The medication is produced by a company called novaplus™ located in Chicago, USA.*
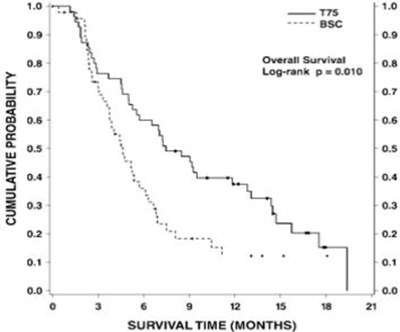
Figure 4 - doc09 0000 05
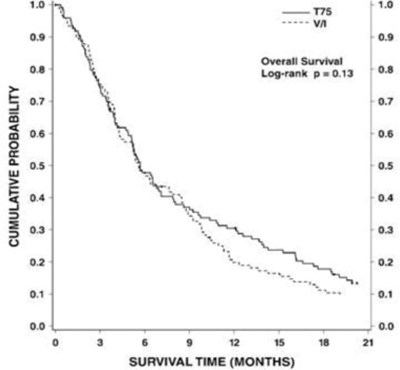
The text describes a graph or chart related to "CUMULATIVE PROBABILITY" with a Y-axis featuring the title "SURVIVAL TIME (MONTHS)" and an X-axis, which is not shown or legible. However, the text is incomplete and lacks any further details, making it difficult to interpret.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.