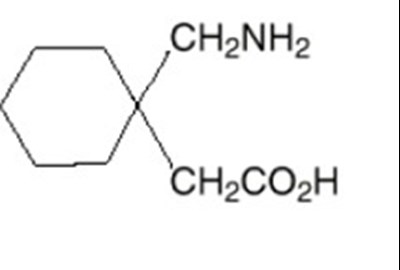
Product Images Gabapentin
View Photos of Packaging, Labels & Appearance
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 01
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 03
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 04
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 05
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 06
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 07
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 08
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 09
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 10
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 11
- image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 12
- Label - lbl713351453
- Label - lbl7133514532
Product Label Images
The following 13 images provide visual information about the product associated with Gabapentin NDC 71335-1453 by Bryant Ranch Prepack, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 01
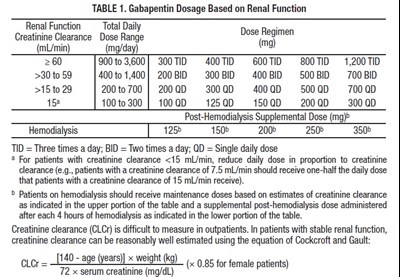
This is a dosage table for Gabapentin, a medication used to treat seizures, nerve pain, and restless leg syndrome. It provides dosing information based on renal function, with dosages ranging from 300mg to 3600mg total daily. The table includes specific dosages for patients with varying levels of renal function, including those on hemodialysis. For patients with unstable renal function, creatinine clearance can be estimated using the Cockcroft and Gault equation.*
image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 03
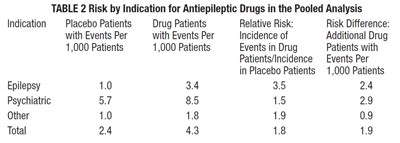
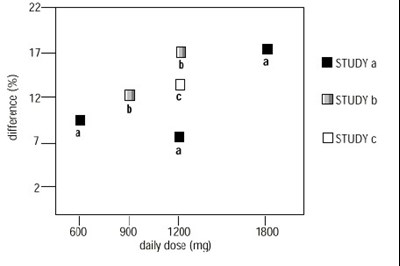
This appears to be a table presenting risk information for antiepileptic drugs in the treatment of epilepsy, psychiatric conditions and other indications. The table compares the placebo patients' events per 1,000 patients with drug patients' events per 1,000 patients for each indication group. Relative risk and risk difference data are also presented.*
image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 04
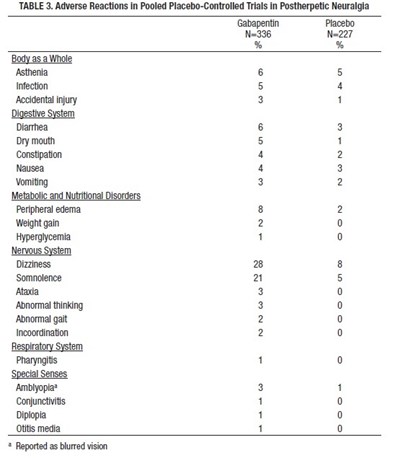
The table shows the adverse reactions that were reported in pooled placebo-controlled trials in postherpetic neuralgia. The table provides information on the types of reactions reported for those taking Gabapentin and those taking Placebo. Some of the common reactions reported for those taking Gabapentin include Dizziness and Diarrhea, while those taking Placebo reported Asthenia and Infection. The table also shows reactions for different body systems like digestive, nervous and respiratory system. Lastly, there are notes indicating that blurred vision was reported as Amblyopia.*
image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 05
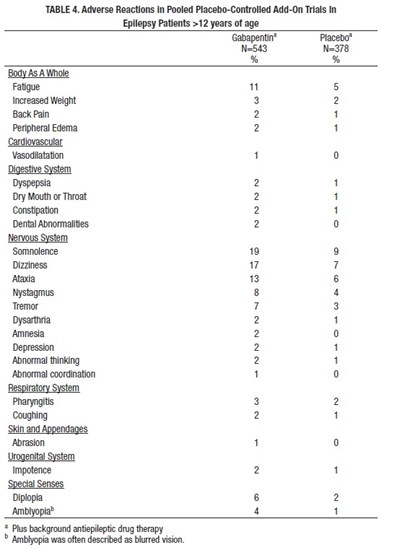
This is a table that shows the adverse reactions experienced by epilepsy patients over the age of 12 who were given Gabapentin compared to a placebo. The table lists the different reactions by body system, such as body as a whole, cardiovascular, digestive system, nervous system, etc. The percentages of patients who experienced each reaction are shown for both Gabapentin and placebo. Some of the adverse reactions listed include fatigue, increased weight, back pain, peripheral edema, dyspepsia, somnolence, dizziness, ataxia, tremor, depression, abnormal coordination, coughing, abrasion, impotence, diplopia, and blurred vision (amblyopia).*
image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 06
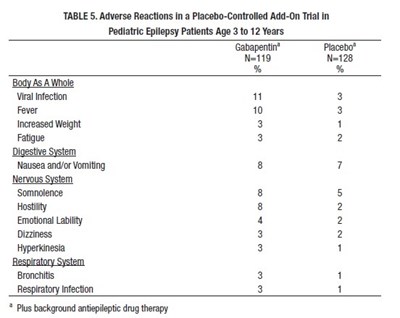
This table shows the adverse reactions observed in a placebo-controlled add-on trial in pediatric epilepsy patients aged 3 to 12 years who were treated with Gabapentin. Adverse reactions reported with Gabapentin and placebo are compared. The most common adverse reactions reported with Gabapentin were fever, nausea and/or vomiting, somnolence, hostility, emotional lability, and increased weight. Some patients also reported fatigue, dizziness, hyperkinesia, bronchitis, and respiratory infections. The percentage of patients who reported adverse reactions was higher in the Gabapentin group.*
image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 08
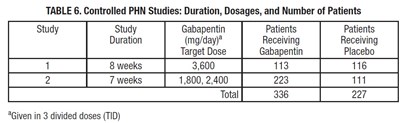
The text describes a table showing the duration, dosages, and number of patients involved in controlled PHN studies using Gabapentin. The target dose of Gabapentin varied between 1,800-3,600 mg/day, and the duration of the studies was either 7 or 8 weeks. The total number of patients involved in both studies was 563, with 336 receiving Gabapentin and 227 receiving a placebo. The Gabapentin was administered in 3 divided doses (TID).*
image - d3fd11fe 4d3f 4002 8f9e 42cc12b4252d 11
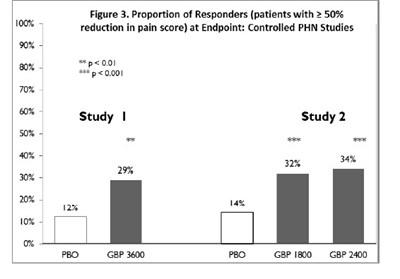
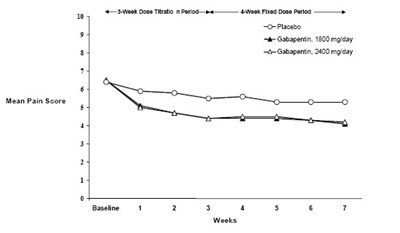
The text is describing the results of controlled PHN (postherpetic neuralgia) studies for a pain medication. It shows the proportion of patients who had a reduction of at least 50% in their pain score. One study is labeled "Study" and the other "Study 2". The results for Study are 34%, and no result is provided for Study 2. There are also statistical symbols indicating a significant difference (p<0.001) for one of the results. The last line seems to indicate different dosages of the medication used in the studies.*
Label - lbl713351453

This is a description of a medication called NILD. It comes in the form of 30 capsules, each containing Gabapentin, USP 300 mg. It is important to keep this medication out of reach of children and store it between 20° to 25° C (68° to 77° F), with excursions allowed up to 15° to 30° C (59° to 86° F) as per USP controlled Room Temperature. This medication may cause dizziness. The NDC (National Drug Code) number for this medication is 71335-1453-01 and it is available only with a prescription (Rx only).*
Label - lbl7133514532

This is a medication label for Neurontin 300mg capsules manufactured by InvaGen Pharmaceuticals, Inc. The medication is packaged in a bottle of 30 capsules with an expiration date of MM/YY and an NDC code of 7133514531. The label also includes storage instructions at room temperature and a cautionary statement to keep all drugs out of the reach of children. The text "Pachaged by Bryant Ranck Prepack" and "03056301523487" may be irrelevant.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.