Product Images Donepezil Hydrochloride
View Photos of Packaging, Labels & Appearance
- Figure 1. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing 24 Weeks of Treatment. - donepezil fig1
- Figure 10. Cumulative Percentage of Patients Completing 6 Months of Double-blind Treatment with Particular Changes from Baseline in ADCS-ADL-Severe Scores. - donepezil fig10
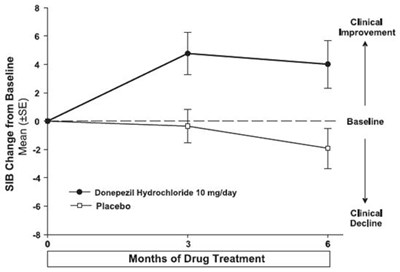
- Figure 11. Time-course of the Change from Baseline in SIB Score for Patients Completing 24 Weeks of Treatment. - donepezil fig11
- Figure 12. Cumulative Percentage of Patients Completing 24 Weeks of Double-blind Treatment with Specified Changes from Baseline SIB Scores. - donepezil fig12
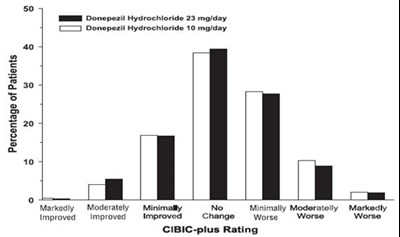
- Figure 13. Frequency Distribution of CIBIC-plus Scores at Week 24. - donepezil fig13
- Figure 2. Cumulative Percentage of Patients Completing 24 Weeks of Double-blind Treatment with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients who Completed the Study were: Placebo 80%, 5 mg/day 85%, and 10 mg/day 68%. - donepezil fig2
- Figure 3. Frequency Distribution of CIBIC-plus Scores at Week 24. - donepezil fig3
- Figure 4. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing the 15-week Study. - donepezil fig4
- Figure 5. Cumulative Percentage of Patients with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients Within Each Treatment Group Who Completed the Study Were: Placebo 93%, 5 mg/day 90%, and 10 mg/day 82%. - donepezil fig5
- Figure 6. Frequency Distribution of CIBIC-plus Scores at Week 12. - donepezil fig6
- Figure 7. Time Course of the Change from Baseline in SIB Score for Patients Completing 6 Months of Treatment. - donepezil fig7
- Figure 8. Cumulative Percentage of Patients Completing 6 Months of Double-blind Treatment with Particular Changes from Baseline in SIB Scores. - donepezil fig8
- Figure 9. Time Course of the Change from Baseline in ADCS-ADL-Severe Score for Patients Completing 6 Months of Treatment. - donepezil fig9
- Chemical Structure - donepezil str
- Label - lbl713352065
Product Label Images
The following 15 images provide visual information about the product associated with Donepezil Hydrochloride NDC 71335-2065 by Bryant Ranch Prepack, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing 24 Weeks of Treatment. - donepezil fig1

Figure 10. Cumulative Percentage of Patients Completing 6 Months of Double-blind Treatment with Particular Changes from Baseline in ADCS-ADL-Severe Scores. - donepezil fig10
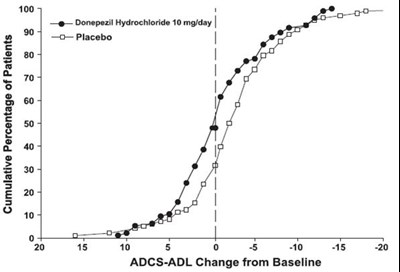
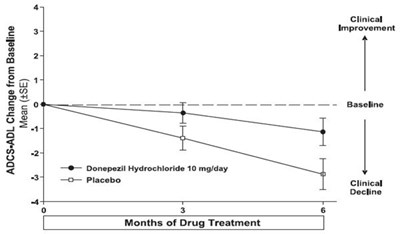
The text is describing a graph showing the cumulative percentage of patients on donepezil hydrochloride 10 mg/day compared to placebo. The graph also shows the change in ADCS-ADL (an assessment tool for Alzheimer’s disease) from baseline.*
Figure 11. Time-course of the Change from Baseline in SIB Score for Patients Completing 24 Weeks of Treatment. - donepezil fig11
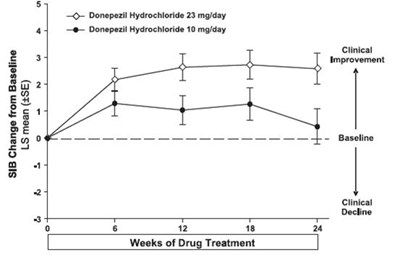
This appears to be a table or chart related to a clinical study comparing two dosages of donepezil hydrochloride (10mg/day and 23mg/day) over a period of 12-18 weeks. The table shows the change from baseline in SIB (Severe Impairment Battery) score, as well as the LS mean and standard error for each dosage. There are categories for clinical improvement and decline. However, the overall context and findings of the study are not readable from this single table excerpt.*
Figure 12. Cumulative Percentage of Patients Completing 24 Weeks of Double-blind Treatment with Specified Changes from Baseline SIB Scores. - donepezil fig12
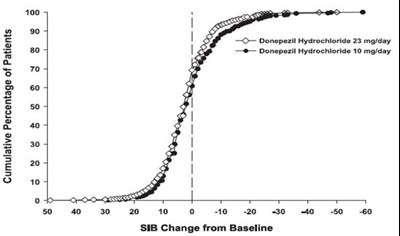
This appears to be a graph displaying the cumulative percentage of patients based on a change from baseline in SIB (Severe Impairment Battery) scores. The x-axis ranges from 0 to 60 with increments of 10, indicating the change from baseline in SIB scores. The y-axis ranges from 0 to 100 and displays the cumulative percentage of patients. It can be deduced that at a score of zero on the x-axis, 100% of the patients had no change from the baseline SIB score. However, without any additional context or information, it is difficult to interpret the exact purpose or meaning of this data.*
Figure 2. Cumulative Percentage of Patients Completing 24 Weeks of Double-blind Treatment with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients who Completed the Study were: Placebo 80%, 5 mg/day 85%, and 10 mg/day 68%. - donepezil fig2
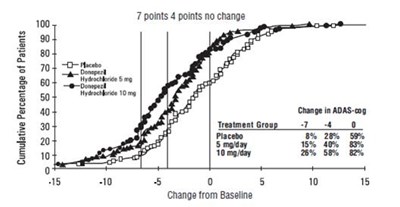
Figure 3. Frequency Distribution of CIBIC-plus Scores at Week 24. - donepezil fig3
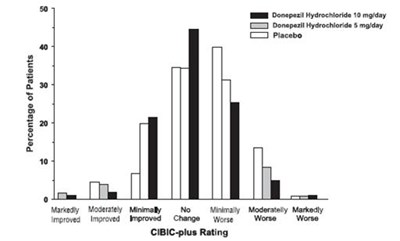
This text appears to be a fragment of a data analysis report related to a medical study. The first section includes data on the percentage of patients that participated in the study, but there seems to be a formatting error as there is no text explaining what this data represents. The second section provides information on the improvement or deterioration of participants' condition, likely related to treatment of hydrochordone. The third section mentions a CIBIC-plus rating, which could be a measure of the complexity or severity of participants' condition. Overall, without more context it is difficult to interpret this text accurately.*
Figure 4. Time-course of the Change from Baseline in ADAS-cog Score for Patients Completing the 15-week Study. - donepezil fig4
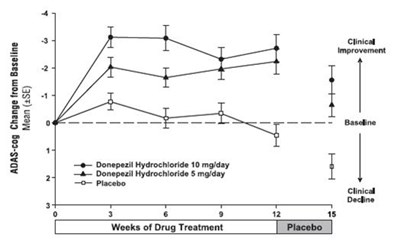
Figure 5. Cumulative Percentage of Patients with Specified Changes from Baseline ADAS-cog Scores. The Percentages of Randomized Patients Within Each Treatment Group Who Completed the Study Were: Placebo 93%, 5 mg/day 90%, and 10 mg/day 82%. - donepezil fig5

Figure 6. Frequency Distribution of CIBIC-plus Scores at Week 12. - donepezil fig6

This appears to be a table showing the percentage of patients who reported improvement or worsening after taking either Donepezil Hydrochloride (at different dosages) or a placebo, as well as their CIBIC-plus rating. There is no further available information to provide a more comprehensive description.*
Figure 7. Time Course of the Change from Baseline in SIB Score for Patients Completing 6 Months of Treatment. - donepezil fig7
This text provides a table comparing the mean change from baseline in SIB scores after three months of drug treatment between patients receiving Donepezil Hydrochloride 10 mg/day and those receiving placebo. It also suggests that the table may include data on clinical improvement but only shows graphical elements which do not convey the specifics of the data.*
Figure 8. Cumulative Percentage of Patients Completing 6 Months of Double-blind Treatment with Particular Changes from Baseline in SIB Scores. - donepezil fig8
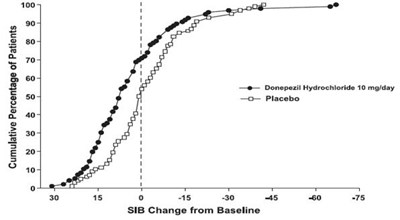
Figure 9. Time Course of the Change from Baseline in ADCS-ADL-Severe Score for Patients Completing 6 Months of Treatment. - donepezil fig9
Label - lbl713352065

This appears to be the package label or description of a medication called "Donepezil 5mg." The tablets are white and round with the imprint "ET59" on them. The medication is comparable to Aricept Smg Tablet and is produced by a company called Elysium Pharmaceuticals Limited. The medication should be stored at room temperature between 20°C-25°C (68°-77°F) and kept out of the reach of children. There is no information available on what the medication is used for or how to take it.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.