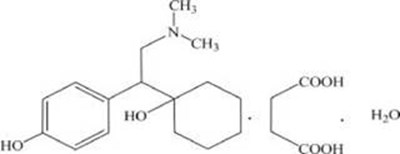
Product Images Desvenlafaxine Succinate
View Photos of Packaging, Labels & Appearance
- Image - e104f9c6 757f 4126 9479 8d50db02fe66 01
- Image - e104f9c6 757f 4126 9479 8d50db02fe66 02
- Image - e104f9c6 757f 4126 9479 8d50db02fe66 03
- Image - e104f9c6 757f 4126 9479 8d50db02fe66 04
- Image - e104f9c6 757f 4126 9479 8d50db02fe66 05
- Image - e104f9c6 757f 4126 9479 8d50db02fe66 06
- Label - lbl713352494
Product Label Images
The following 7 images provide visual information about the product associated with Desvenlafaxine Succinate NDC 71335-2494 by Bryant Ranch Prepack, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Image - e104f9c6 757f 4126 9479 8d50db02fe66 02

The text provides information on the impact of intrinsic factors such as renal, hepatic impairment, and population description on the pharmacokinetics of Desvenlafaxine. It includes data on geometric mean ratios for different parameters like Cmax and AUC under various conditions like moderate and severe impairment, end-stage renal disease (ESRD), hepatic impairment, and across different population descriptions like gender. The text suggests that these factors can influence the pharmacokinetics of Desvenlafaxine.*
Image - e104f9c6 757f 4126 9479 8d50db02fe66 03
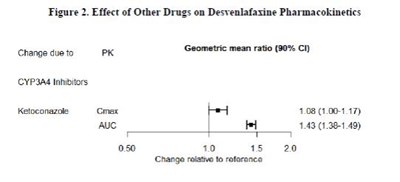
This text provides information on the effect of other drugs on Desvenlafaxine pharmacokinetics, specifically looking at CYP3A4 Inhibitors. The geometric mean ratio and 90% confidence interval for Ketoconazole are shown, along with the values for Gmex and AuC. The data suggests a potential impact of Ketoconazole on Desvenlafaxine pharmacokinetics.*
Image - e104f9c6 757f 4126 9479 8d50db02fe66 04

This text provides information on the effects of Desvenlafaxine on the pharmacokinetics of other drugs. It includes data on the changes in geometric mean ratios for various substrates of CYP2D6, CYP3A, and CYP3M enzymes when interacting with Desvenlafaxine at different dosages. The text also presents results for active metabolites of Tamoxifen and Aripiprazole. The table shows how different doses of Desvenlafaxine impact the pharmacokinetics of these drugs.*
Image - e104f9c6 757f 4126 9479 8d50db02fe66 05

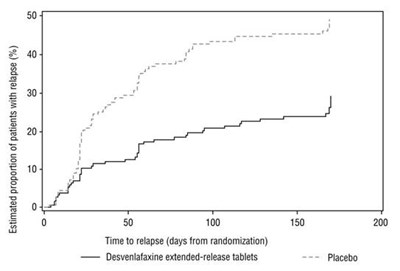
This figure shows the estimated proportion of relapses in a study comparing Desvenlafaxine extended-release tablets (50 mg) to a placebo over time (in days from randomization). The graph indicates a higher percentage of relapses in the placebo group compared to the Desvenlafaxine group.*
Label - lbl713352494

This is a description of Desvenlafaxine Extended-Release Tablets. Each film-coated tablet contains 38mg of Desvenlafaxine Succinate equivalent to 25mg of Desvenlafaxine. It is advised to keep the tablets out of reach of children and store them at temperatures between 20°C to 25°C. Swallow the tablets whole and do not break, crush, dissolve, or chew them. For more information, it is recommended to consult the Patient Package Inserts and Medication Guide provided by the pharmacist. The tablets are meant for prescription use only and come in a pack of 30 tablets. The tablets are repackaged by Bryant Ranch Prepack, Inc. and manufactured by Lupin Limited. For further details, the NDC number is provided for reference.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.