Product Images Xipere
View Photos of Packaging, Labels & Appearance
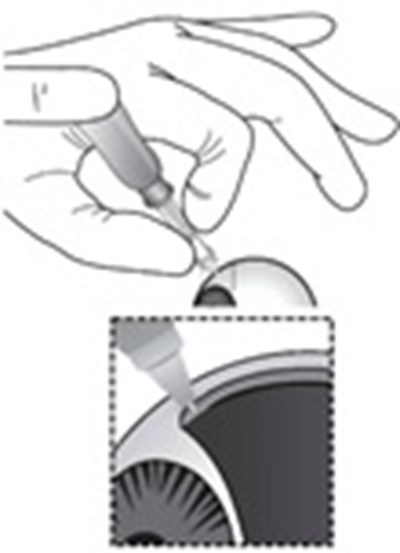
- Components for Administration - xipere 01
- Step 1 - xipere 02
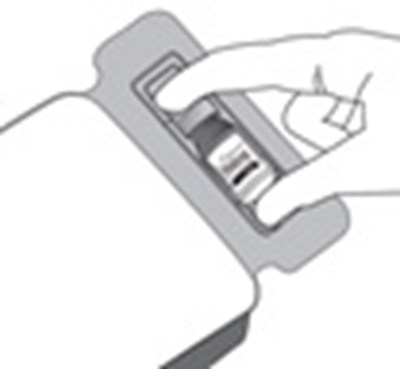
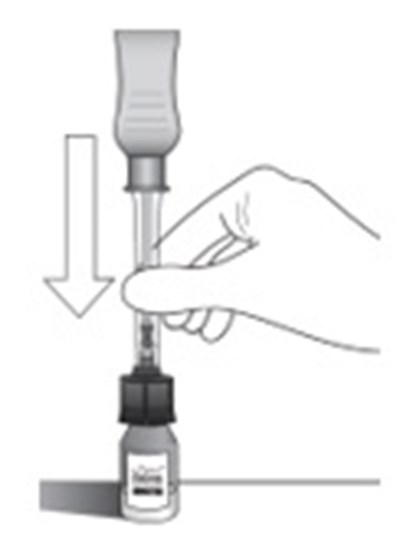
- Step 2 - xipere 03
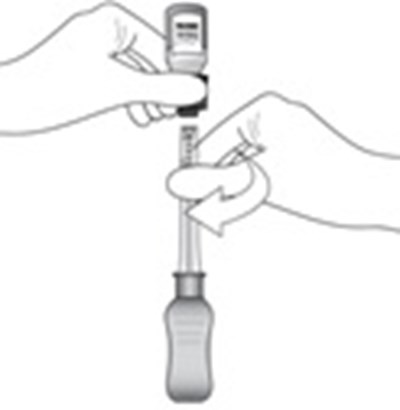
- Step 3 - xipere 04
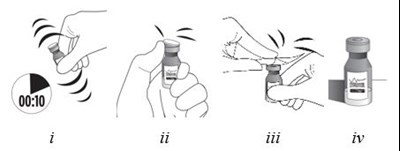
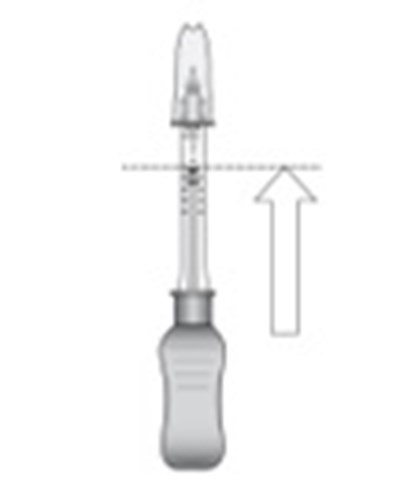
- Step 4 - xipere 05
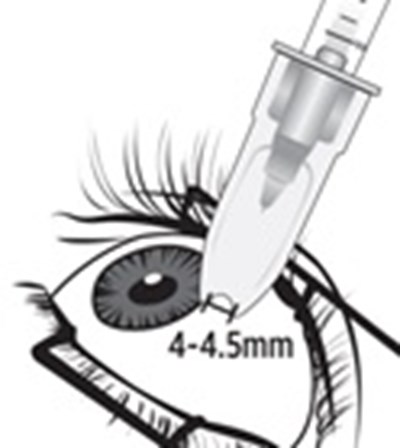
- Step 5 - xipere 06
- Step 6 - xipere 07
- Step 7 - xipere 08
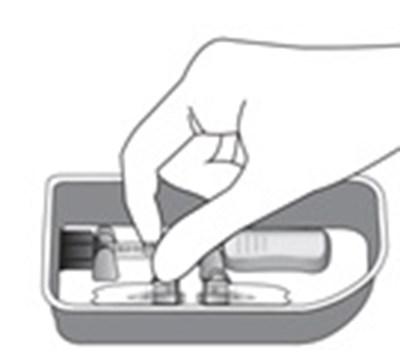
- Step 8 - xipere 09
- Step 9 - xipere 10
- Step 10 - xipere 11
- Step 11 - xipere 12
- Step 12 - xipere 13
- Step 13 - xipere 14
- Step 14 - xipere 15
- Step 15 - xipere 16
- Step 16 - xipere 17
- Chemical Structure - xipere 18
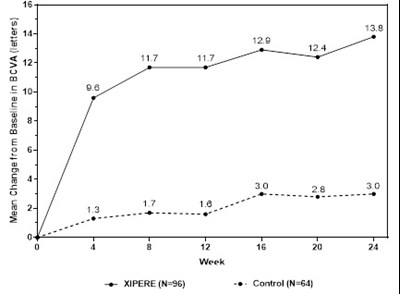
- Figure 1 - xipere 19
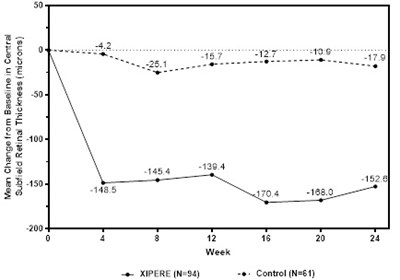
- Figure 2 - xipere 20
- Vial Label - xipere 21
- Lid Label - xipere 22
- Carton Label - xipere 23
Product Label Images
The following 23 images provide visual information about the product associated with Xipere NDC 71565-040 by Clearside Biomedical, Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Vial Label - xipere 21

NDC 71565-040-25 is a code for a single dose of XIPERFE, an injectable suspension containing 40 mg/mL of triamcinolone acetonide. This medication is intended for use in the suprachoroidal space and is manufactured for Clearside Biomedical, Inc. The text is sterile and has the product code ROl 09l. No other information could be obtained from the text except for a random string of characters at the end.*
Lid Label - xipere 22

XIPERE is a medication used for suprachoroidal use, containing triamcinolone acetonide injectable suspension at a concentration of 40mg per mL. It is supplied in a sterile form and each sealed tray contains a microinjector syringe, vial adapter, and two needles of different lengths. XIPERE is manufactured by Clearside Biomedical, Inc. and is available by prescription only. More information about XIPERE can be found on the Clearside Biomedical website. Lot number and other information are also included on the packaging.*
Carton Label - xipere 23

XIPERE is a triamcinolone acetonide injectable suspension 40 mg/mL designed for suprachoroidal use. The package includes a single-dose glass vial of the suspension, an SCS Microinjector® syringe with the vial adapter attached, a 30-G x 900-um needle, a 30-G x 1100-um needle, and a package insert. The text also includes manufacturing and distribution information.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.