Product Images Entresto
View Photos of Packaging, Labels & Appearance
- 71610-807-37 - 71610 807 37
- Aphena - Aphena
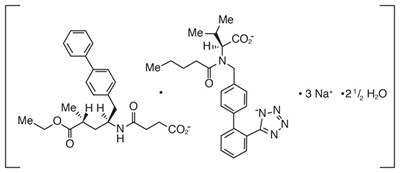
- Valsartan structural formula - ENTRESTO 01
- Figure 1: Effect of ENTRESTO on Pharmacokinetics of Coadministered Drugs - ENTRESTO 02
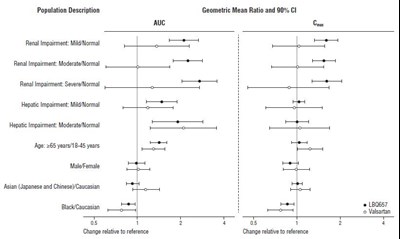
- Figure 2: Pharmacokinetics of ENTRESTO in Specific Populations - ENTRESTO 03
- Figure 3: Kaplan-Meier Curves for the Primary Composite Endpoint (A), Cardiovascular Death (B), and Heart Failure Hospitalization (C) - ENTRESTO 04
- Figure 4: Primary Composite Endpoint (CV Death or HF Hospitalization) - Subgroup Analysis - ENTRESTO 05
- Figure 5: Mean Number of Events Over Time for the Primary Composite Endpoint of Total HF Hospitalizations and CV Death - ENTRESTO 09
- Figure 6: Primary Composite Endpoint of Total HF Hospitalizations and CV Death – Subgroup Analysis (PARAGON-HF) - ENTRESTO 10
- Figure 7: Treatment Effect for the Composite Endpoint of Time to First HF Hospitalization or CV Death by LVEF in PARADIGM-HF and PARAGON-HF - ENTRESTO 11
Product Label Images
The following 10 images provide visual information about the product associated with Entresto NDC 71610-807 by Aphena Pharma Solutions - Tennessee, Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1: Effect of ENTRESTO on Pharmacokinetics of Coadministered Drugs - ENTRESTO 02

This is not a readable text.*
Figure 3: Kaplan-Meier Curves for the Primary Composite Endpoint (A), Cardiovascular Death (B), and Heart Failure Hospitalization (C) - ENTRESTO 04

Figure 4: Primary Composite Endpoint (CV Death or HF Hospitalization) - Subgroup Analysis - ENTRESTO 05

This is a dataset containing various demographic and health-related information such as age, gender, weight, race, region, NYHA class, estimated GFR, diabetes status, blood pressure, ejection fraction, atrial fibrillation, NT-proBNP levels, hypertension status, prior medication use, prior hospitalization for heart failure, time since diagnosis, cause of heart failure, and presence of ICD including CRT-D. Additionally, there are numbers related to the use of medications like Entresto and Enalapril, showing the percentage of participants falling into different categories or experiencing different outcomes. The dataset also includes hazard ratios with confidence intervals comparing the efficacy of Entresto and Enalapril.*
Figure 5: Mean Number of Events Over Time for the Primary Composite Endpoint of Total HF Hospitalizations and CV Death - ENTRESTO 09

Figure 6: Primary Composite Endpoint of Total HF Hospitalizations and CV Death – Subgroup Analysis (PARAGON-HF) - ENTRESTO 10

Figure 7: Treatment Effect for the Composite Endpoint of Time to First HF Hospitalization or CV Death by LVEF in PARADIGM-HF and PARAGON-HF - ENTRESTO 11

This is useful information comparing hazard ratios for PARADIGM-HF and PARAGON-HF clinical trials evaluating the use of ENTRESTO against enalapril and valsartan, respectively. The diagram displays left ventricular ejection fraction at screening (%) along with hazard ratios for different scenarios.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.