Product Images Entresto
View Photos of Packaging, Labels & Appearance
- Label - 71610 810 32
- Aphena - Aphena
- Valsartan structural formula - ENTRESTO 01
- Figure 1: Effect of ENTRESTO on Pharmacokinetics of Coadministered Drugs - ENTRESTO 02
- Figure 2: Pharmacokinetics of ENTRESTO in Specific Populations - ENTRESTO 03
- Figure 3: Kaplan-Meier Curves for the Primary Composite Endpoint (A), Cardiovascular Death (B), and Heart Failure Hospitalization (C) - ENTRESTO 04
- Figure 4: Primary Composite Endpoint (CV Death or HF Hospitalization) - Subgroup Analysis - ENTRESTO 05
- Figure 5: Mean Number of Events Over Time for the Primary Composite Endpoint of Total HF Hospitalizations and CV Death - ENTRESTO 09
- Figure 6: Primary Composite Endpoint of Total HF Hospitalizations and CV Death – Subgroup Analysis (PARAGON-HF) - ENTRESTO 10
- Figure 7: Treatment Effect for the Composite Endpoint of Time to First HF Hospitalization or CV Death by LVEF in PARADIGM-HF and PARAGON-HF - ENTRESTO 11
Product Label Images
The following 10 images provide visual information about the product associated with Entresto NDC 71610-810 by Aphena Pharma Solutions - Tennessee, Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1: Effect of ENTRESTO on Pharmacokinetics of Coadministered Drugs - ENTRESTO 02
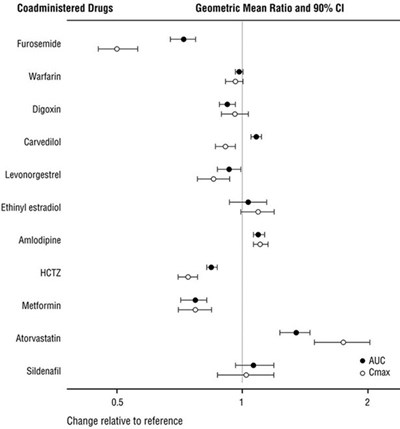
This text provides information on the geometric mean ratio and 90% confidence interval for several drugs when coadministered with other drugs. The drugs mentioned include Furosemide, Warfarin, Digoxin, Carvedilol, Levonorgestrel, Ethinyl estradiol, Amlodipine, HCTZ, Metformin, Atorvastatin, and Sildenafil. The text suggests a comparison of the drugs' effects when taken together.*
Figure 3: Kaplan-Meier Curves for the Primary Composite Endpoint (A), Cardiovascular Death (B), and Heart Failure Hospitalization (C) - ENTRESTO 04
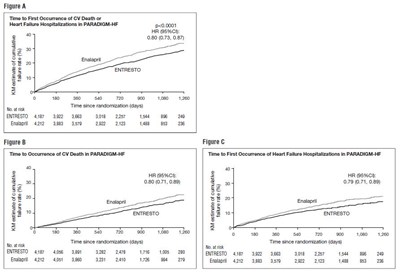
Figure 4: Primary Composite Endpoint (CV Death or HF Hospitalization) - Subgroup Analysis - ENTRESTO 05
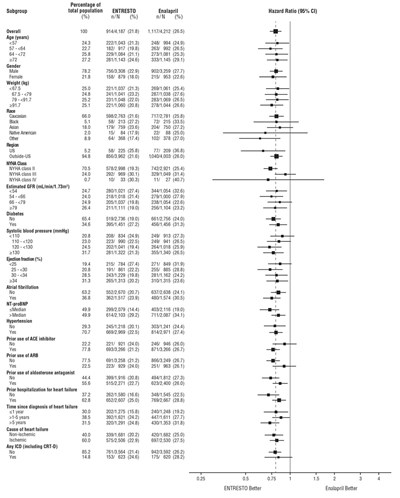
This dataset provides detailed information on various subgroups within a population, including demographics such as age, gender, weight, race, region, and medical conditions like diabetes, hypertension, atrial fibrillation, and heart failure. The data also presents the percentage of individuals in each category and compares outcomes related to the use of medications like Entresto and Enalapril, showcasing hazard ratios for each. These statistics can be valuable for analyzing the impact of different factors on health outcomes in a specific population.*
Figure 5: Mean Number of Events Over Time for the Primary Composite Endpoint of Total HF Hospitalizations and CV Death - ENTRESTO 09
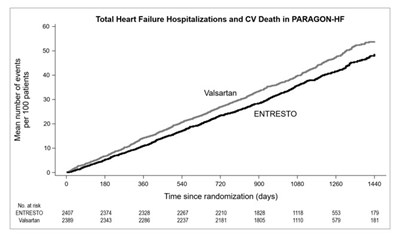
Total Heart Failure Hospitalizations and Cardiovascular Death in PARAGON-HF study with Valsartan and ENTRESTO. The mean number of events per 100 patients is 8. The graph shows the time since randomization (days) and the corresponding data points related to the study.*
Figure 6: Primary Composite Endpoint of Total HF Hospitalizations and CV Death – Subgroup Analysis (PARAGON-HF) - ENTRESTO 10

Figure 7: Treatment Effect for the Composite Endpoint of Time to First HF Hospitalization or CV Death by LVEF in PARADIGM-HF and PARAGON-HF - ENTRESTO 11

This text shows a comparison of Hazard Ratios for ENTRESTO versus enalapril and valsartan in the PARADIGM-HF and PARAGON-HF trials based on left ventricular ejection fraction values. The graph displays the hazard ratios at various left ventricular ejection fraction percentages. This information is valuable for evaluating the efficacy of ENTRESTO in comparison to traditional heart failure medications.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.



