FDA Label for Harris Teeter Foaming
View Indications, Usage & Precautions
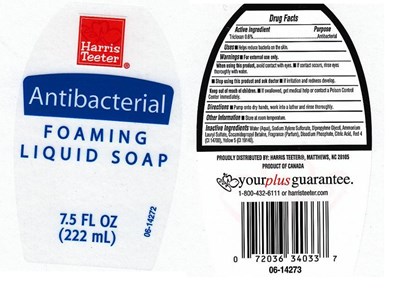
Harris Teeter Foaming Product Label
The following document was submitted to the FDA by the labeler of this product Harris Teeter. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Active Ingredient
Triclosan 0.6%
Purpose
Antibacterial
Warnings
For external use only.
When Using This Product
- Avoid contact with eyes. If contact occurs, rinse with water.
Stop Using This Product And Ask Doctor If
- irritation or redness develops and lasts.
Keep Out Of Reach Of Children
In case of accidental ingestion, get medical help or contact a Poison Control Center immediately.
Directions
Pump onto dry hands, work vigorously into a lather and rinse thoroughly.
Uses
Helps to decrease bacteria on the skin
Inactive Ingredients
Water (Aqua), Sodium Xylenesulfonate, Dipropylene Glycol, Ammonium Lauryl Sulfate, Cocamidopropyl Betaine, Fragrance(Parfum), Disodium Phosphate, Citric Acid,Yellow 5 (CI 19140), Red 40 (CI 16035).
Package Front And Back Labels
ht.jpg
* Please review the disclaimer below.