Product Images Olpruva
View Photos of Packaging, Labels & Appearance
- Structural Formula - olp00 0003 01
- Acer Therapeutics Logo - olp00 0003 02
- Acer Therapeutics Logo - olp00 0003 03
- Figure A - olp00 0003 04
- Figure B - olp00 0003 05
- Figure C - olp00 0003 06
- Figure D - olp00 0003 07
- Figure E - olp00 0003 08
- Figure F - olp00 0003 09
- Figure G - olp00 0003 10
- Figure H - olp00 0003 11
- Figure I - olp00 0003 12
- Figure J - olp00 0003 13
- Figure K - olp00 0003 14
- Acer Therapeutics Logo - olp00 0003 15
- Principal Display Panel – Mix-Aid Label - olp00 0003 16
- Principal Display Panel - 2 g Carton Label - olp00 0003 17
- Principal Display Panel - 2 g Pouch Label - olp00 0003 18
- Principal Display Panel - 2 g Envelope Label - olp00 0003 19
- Principal Display Panel - 3 g Carton Label - olp00 0003 20
- Principal Display Panel - 3 g Pouch Label - olp00 0003 21
- Principal Display Panel - 3 g Envelope Label - olp00 0003 22
- Principal Display Panel - 4 g Carton Label - olp00 0003 23
- Principal Display Panel - 4 g Envelope Label - olp00 0003 24
- Principal Display Panel - 5 g Carton Label - olp00 0003 25
- Principal Display Panel - 5 g Envelope Label - olp00 0003 26
- Principal Display Panel - 6 g Carton Label - olp00 0003 27
- Principal Display Panel - 6 g Envelope Label - olp00 0003 28
- Principal Display Panel - 6.67 g Carton Label - olp00 0003 29
- Principal Display Panel - 6.67 g Envelope Label - olp00 0003 30
- Principal Display Panel - 3.67 g Pouch Label - olp00 0003 31
Product Label Images
The following 31 images provide visual information about the product associated with Olpruva NDC 72542-500 by Acer Therapeutics Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Principal Display Panel – Mix-Aid Label - olp00 0003 16

Mix-Aid is a suspending agent designed to be used with OLPRUVA™, an oral suspension medication containing sodium phenylbutyrate. The packet content of 3.1 grams should be mixed according to patient instructions, which will make the water thicker, allowing OLPRUVA™ to be suspended. The ingredients include food starch-modified, and the nutritional value of one packet is low. Acer Therapeutics Inc. is the manufacturer, and OLPRUVA™ is their trademark. The lot number is 2IT004350, and the product expiry date is not specified.*
Principal Display Panel - 2 g Carton Label - olp00 0003 17

Description: OLPRUVA is a sodium phenylbutyrate oral suspension medication that comes in 50 dosage envelopes. Each envelope contains one 2g packet and is equivalent to 2 grams of sodium phenylbutyrate or 1.75 grams of phenylbutyrate. The recommended dosage and prescribing information are included in the accompanying patient instructions. The medication should be stored at a temperature between 15°C and 30°C. It is important to keep OLPRUVA out of reach of children.*
Principal Display Panel - 2 g Pouch Label - olp00 0003 18
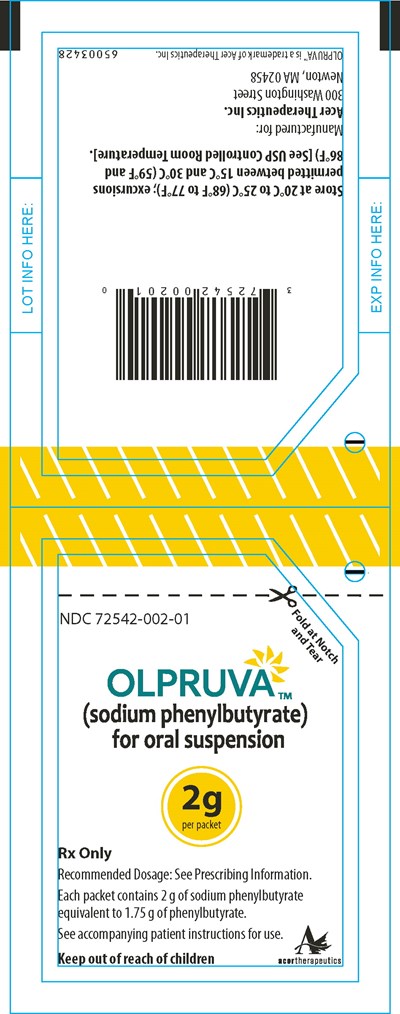
The given text contains lot information and expiry information, which includes the lot number, location, and lot details. Also, it has the details regarding OLPRUVA, a medicine recommended for oral intake. The medicine comes in packets and each packet contains 2 g of medicine, the instructions for the medicine are available in the accompanying patient instructions. Recommended dosage of the medicine is available in the prescribing information.*
Principal Display Panel - 3 g Carton Label - olp00 0003 20

OLPRUVA is an oral suspension medication that contains sodium phenylbutyrate. It is available in dosage envelopes, with each envelope containing 3 grams of sodium phenylbutyrate, which is equivalent to 0.263 grams of phenylbutyrate. The recommended dosage for OLPRUVA is not available and should be prescribed by a healthcare provider. The medication should be stored at room temperature between 15°C and 30°C and kept out of reach of children. Accompanying patient instructions should be consulted for use.*
Principal Display Panel - 3 g Pouch Label - olp00 0003 21
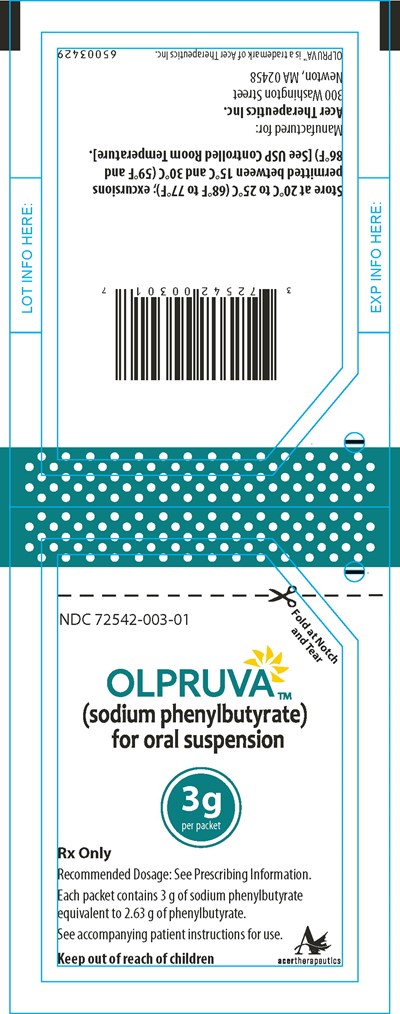
This appears to be a lot information containing a variety of alphanumeric codes and data related to a medication called "sodium phenylbutyrate" which is prescribed as oral suspension. The recommended dosage information is not available. The lot contains packets priced at $39 each and each packet consists of 3 g of sodium phenylbutyrate equivalent to 2.63 g of phenylbutyrate. The text warns to keep it out of reach of children and there is an indication to see the accompanying patient instructions for use. There is no explicit mention of the intended use or medical condition for which the medication is being prescribed.*
Principal Display Panel - 4 g Carton Label - olp00 0003 23

OLPRUVA is a medication in the form of oral suspension that contains sodium phenylbutyrate. It comes in envelopes and each envelope contains 27g of OLPRUVA with one MOH recipient. The recommended dosage is not available and it is important to consult a physician before use. OLPRUVA should be stored at room temperature between 20°C to 25°C (68°F to 77°F). It is important to keep out of reach of children.*
Principal Display Panel - 4 g Envelope Label - olp00 0003 24

Each 4 g of sodium phenylbutyrate is equivalent to 3.51 g of phenylbutyrate. OLPRUVA is a medication available in the form of oral suspension and each envelope includes two 2-gram packets of OLPRUVA and one Mix-Aid packet. The patient instructions for use should be read before use. The recommended dosage needs to be followed and it is essential to keep the medication out of the reach of children. The NDC number of OLPRUVA is 72542-400-02.*
Principal Display Panel - 5 g Carton Label - olp00 0003 25

OLPRUVA is a medication in the form of an oral suspension containing sodium phenylbutyrate. Its NDC number is 7254250018.*
Principal Display Panel - 5 g Envelope Label - olp00 0003 26

This is a description of OLPRUVA, a medication consisting of sodium phenylbutyrate for oral suspension. Each envelope contains a 2 gram packet, a 3 gram packet, and a Mix-Aid packet. It is important to note that 5 grams of sodium phenylbutyrate is equivalent to 4.38 grams of phenylbutyrate. The medication comes with patient instructions for use and is only available with a prescription.*
Principal Display Panel - 6 g Carton Label - olp00 0003 27

OLPRUVA is a medication in the form of sodium phenylbutyrate for oral suspension. The provided text also includes a product code (NDC 7254260018) but does not provide any additional information.*
Principal Display Panel - 6 g Envelope Label - olp00 0003 28

OLPRUVA is a sodium phenylbutyrate oral suspension medication that comes in envelopes containing two OLPRUVA 3 gram packets and one Mix-Aid packet. Each 6 g of sodium phenylbutyrate is equivalent to 5.26 g of phenylbutyrate. The recommended dosage and patient instructions for use should be obtained from the prescribing information, and it should be kept out of reach of children.*
Principal Display Panel - 6.67 g Carton Label - olp00 0003 29

OLPRUVA is a sodium phenylbutyrate medication used for oral suspension. The NDC code is 7254266718, and it should be kept out of reach of children.*
Principal Display Panel - 6.67 g Envelope Label - olp00 0003 30

OLPRUVA is a sodium phenylbutyrate medication available for oral consumption. Each envelope of OLPRUVA consists of a 3 grams packet, a 3.67 grams packet, and a Mix-Aid packet. The equivalent measure of 6.67 grams of sodium phenylbutyrate is 5.85 grams of phenylbutyrate. Patient instructions for use are provided. The recommended dosage should be determined by a healthcare professional, and the medication should be kept out of reach of children.*
Principal Display Panel - 3.67 g Pouch Label - olp00 0003 31

This text contains specific information about a medication called OLPRUVA, which is an oral suspension of sodium phenylbutyrate. Each packet contains 3.67 g of sodium phenylbutyrate equivalent to 3.22 g of phenylbutyrate. The recommended dosage is not provided in this text, and readers are instructed to see the prescribing information. The text also provides an NDC number and mentions the accompanying patient instructions for use. Additionally, an expiration date is referenced but not explicitly provided.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.
















