Product Images Oxcarbazepine
View Photos of Packaging, Labels & Appearance
- oxcarbazepinetabfig1 - oxcarbazepinetabfig1
- oxcarbazepinetabfig2 - oxcarbazepinetabfig2
- oxcarbazepinetabfig3 - oxcarbazepinetabfig3
- oxcarbazepinetabfig4 - oxcarbazepinetabfig4
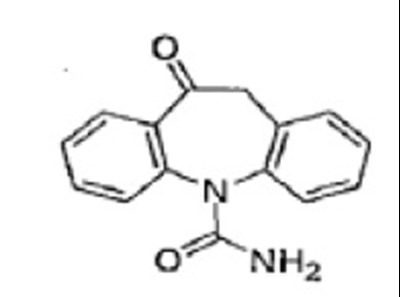
- oxcarbazepinetabstruct - oxcarbazepinetabstruct
- oxcarbazipinetab150mglabel - oxcarbazipinetab150mglabel
- oxcarbazipinetab300mglabel - oxcarbazipinetab300mglabel
- oxcarbazipinetab600mglabel - oxcarbazipinetab600mglabel
Product Label Images
The following 8 images provide visual information about the product associated with Oxcarbazepine NDC 72865-284 by Xlcare Pharmaceuticals Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
oxcarbazepinetabfig1 - oxcarbazepinetabfig1

A log-rank test was conducted, resulting in a p-value of 0.0001 for the comparison between two groups under treatment with Oxcarbazepine. This statistical analysis assesses the difference in survival times or time-to-event data.*
oxcarbazepinetabfig2 - oxcarbazepinetabfig2

Days from 1st dose to 1st seizure can be evaluated for a treatment group receiving Oxcarbazepine compared to a placebo group. The graph appears to show a timeline with days on the horizontal axis.*
oxcarbazepinetabfig3 - oxcarbazepinetabfig3

This text provides results from a log-rank test, showing a p-value of 0.0001. It also presents a table showing "Time to exit (days)" for a treatment group labeled as "HIGH - LOW". The table includes intervals from 10 to 130 days, indicating the data analyzed in the log-rank test.*
oxcarbazepinetabfig4 - oxcarbazepinetabfig4

This description provides the results of a log-rank test comparing two groups (HIGH and LOW) in terms of time to exit (in days). The test resulted in a p-value of 0.0001, indicating a statistically significant difference between the two groups.*
oxcarbazipinetab150mglabel - oxcarbazipinetab150mglabel

XLcare is a medication containing 150 mg of oxcarbazepine USP. It is a film-coated tablet commonly used to treat certain conditions. The dosage information can be found in the package insert. The tablets are manufactured for distribution by Evaric Pharmaceuticals Inc., based in Hauppauge, New York. For more details or assistance, one can contact the manufacturer at the provided phone number. Always keep this medication in a tightly closed container and out of reach of children.*
oxcarbazipinetab300mglabel - oxcarbazipinetab300mglabel

This text provides information about XLcare Oxcarbazepine Tablets, USP, in a 300 mg dosage form. The medication is intended for prescription use only, with a Medication Guide accompanying each patient's supply. The tablets are film-coated and should be stored within a specific temperature range. The manufacturer details are included with contact information for further inquiries.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.