FDA Label for Motpoly Xr
View Indications, Usage & Precautions
- 1.1 PARTIAL-ONSET SEIZURES
- 2.1 DOSAGE INFORMATION
- 2.2 CONVERTING FROM A SINGLE ANTIEPILEPTIC (AED) TO MOTPOLY XR MONOTHERAPY FOR THE TREATMENT OF PARTIAL-ONSET SEIZURES
- 2.3 DOSAGE INFORMATION FOR PATIENTS WITH RENAL IMPAIRMENT
- OTHER
- 2.4 DOSAGE INFORMATION FOR PATIENTS WITH HEPATIC IMPAIRMENT
- 2.5 ADMINISTRATION INSTRUCTIONS FOR MOTPOLY XR CAPSULES
- 2.6 DISCONTINUATION OF MOTPOLY XR
- 4 CONTRAINDICATIONS
- 5.1 SUICIDAL BEHAVIOR AND IDEATION
- 5.2 DIZZINESS AND ATAXIA
- 5.4 SYNCOPE
- 5.5 WITHDRAWAL OF ANTIEPILEPTIC DRUGS (AEDS)
- 5.6 DRUG REACTION WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS (DRESS)/MULTI-ORGAN HYPERSENSITIVITY
- 6 ADVERSE REACTIONS
- 6.1 CLINICAL TRIALS EXPERIENCE
- 6.2 POSTMARKETING EXPERIENCE
- 7.1 STRONG CYP3A4 OR CYP2C9 INHIBITORS
- 7.2 CONCOMITANT MEDICATIONS THAT AFFECT CARDIAC CONDUCTION
- 7.3 CNS DEPRESSANTS
- 8.5 GERIATRIC USE
- 8.6 RENAL IMPAIRMENT
- 8.7 HEPATIC IMPAIRMENT
- 9.1 CONTROLLED SUBSTANCE
- 9.2 ABUSE
- 9.3 DEPENDENCE
- 10 OVERDOSAGE
- 11 DESCRIPTION
- 12.1 MECHANISM OF ACTION
- 12.2 PHARMACODYNAMICS
- 12.3 PHARMACOKINETICS
- 12.5 PHARMACOGENOMICS
- 14.1 MONOTHERAPY IN PATIENTS WITH PARTIAL-ONSET SEIZURES
- 14.2 ADJUNCTIVE THERAPY IN PATIENTS WITH PARTIAL-ONSET SEIZURES
- 16.2 STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- SPL MEDGUIDE
- PRINCIPAL DISPLAY PANEL - 100 MG CAPSULE BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - 150 MG CAPSULE BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - 200 MG CAPSULE BOTTLE LABEL
- PRINCIPAL DISPLAY PANEL - PHYSICIAN SAMPLES
Motpoly Xr Product Label
The following document was submitted to the FDA by the labeler of this product Aucta Pharmaceuticals, Inc.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
1.1 Partial-Onset Seizures
MOTPOLY XR is indicated for the treatment of partial-onset seizures in adults and in pediatric patients weighing at least 50 kg.
2.1 Dosage Information
The recommended dosage for monotherapy and adjunctive therapy for partial-onset seizures in adults and in pediatric patients weighing at least 50 kg is included in Table 1. Dosage should be increased based on clinical response and tolerability, no more frequently than once per week. Titration increments should not exceed those shown in Table 1.
| Age and Body Weight | Initial Dosage | Titration Regimen | Maintenance Dosage |
|---|---|---|---|
| Adults (17 years and older) | Monotherapy**:
200 mg once daily Adjunctive Therapy: 100 mg once daily | Increase by 100 mg once daily every week | Monotherapy**: 300 mg to 400 mg once daily Adjunctive Therapy: 200 mg to 400 mg once daily |
| Pediatric patients weighing at least 50 kg | 100 mg once daily | Increase by 100 mg once daily every week | Monotherapy**: 300 mg to 400 mg once daily Adjunctive Therapy: |
*when not specified, the dosage is the same for monotherapy for partial-onset seizures and adjunctive therapy for partial-onset seizures.
**Monotherapy for partial-onset seizures only
In adjunctive clinical trials in adult patients with partial-onset seizures, a dosage higher than 400 mg per day was not more effective and was associated with a substantially higher rate of adverse reactions [see Adverse Reactions (6.1) and Clinical Studies (14.2)] .
2.2 Converting From A Single Antiepileptic (Aed) To Motpoly Xr Monotherapy For The Treatment Of Partial-Onset Seizures
For patients who are already on a single AED and will convert to MOTPOLY XR monotherapy, withdrawal of the concomitant AED should not occur until the therapeutic dosage of MOTPOLY XR is achieved and has been administered for at least 4 days. A gradual withdrawal of the concomitant AED over at least 6 weeks is recommended.
2.3 Dosage Information For Patients With Renal Impairment
For patients with mild to moderate renal impairment, no dosage adjustment is necessary.
For patients with severe renal impairment [creatinine clearance (CL
CR) less than 30 mL/min as estimated by the Cockcroft-Gault equation for adults; CL
CR less than 30 mL/min/1.73m
2 as estimated by the Schwartz equation for pediatric patients] or end-stage renal disease, the maximum recommended dosage is 300 mg.
In all patients with renal impairment, dose initiation and titration should be based on clinical response and tolerability.
Other
Hemodialysis
MOTPOLY XR is effectively removed from plasma by hemodialysis. Following a 4-hour hemodialysis treatment, dosage supplementation of up to 50% should be considered.
Concomitant Strong CYP3A4 or CYP2C9 Inhibitors
Dose reduction may be necessary in patients with renal impairment who are taking strong inhibitors of CYP3A4 and CYP2C9 [see Drug Interactions (7.1), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)] .
Concomitant Strong CYP3A4 and CYP2C9 Inhibitors
Dose reduction may be necessary in patients with hepatic impairment who are taking strong inhibitors of CYP3A4 and CYP2C9 [see Drug Interactions (7.1), Use in Specific Populations (8.7), and Clinical Pharmacology (12.3)] .
MOTPOLY XR extended-release capsules contain white or off-white beads and are available in the following strengths:
- 100 mg: Yellow opaque cap with “A” printed in black ink and a white opaque body with “100” printed in black ink
- 150 mg: Brown opaque cap with “A” printed in black ink and a white opaque body with “150” printed in black ink
- 200 mg: Blue opaque cap with “A” printed in black ink and a white opaque body with “200” printed in black ink
- Drug interaction studies with AEDs
- Effect of Lacosamide on concomitant AEDs
Lacosamide 400 mg/day had no influence on the pharmacokinetics of 600 mg/day valproic acid and 400 mg/day carbamazepine in healthy subjects.
The placebo-controlled clinical studies in patients with partial-onset seizures showed that steady-state plasma concentrations of levetiracetam, carbamazepine, carbamazepine epoxide, lamotrigine, topiramate, oxcarbazepine monohydroxy derivative (MHD), phenytoin, valproic acid, phenobarbital, gabapentin, clonazepam, and zonisamide were not affected by concomitant intake of lacosamideat any dose. - Effect of concomitant AEDs on Lacosamide
Drug-drug interaction studies in healthy subjects showed that 600 mg/day valproic acid had no influence on the pharmacokinetics of 400 mg/daylacosamide. Likewise, 400 mg/day carbamazepine had no influence on the pharmacokinetics of lacosamidein a healthy subject study. Population pharmacokinetics results in patients with partial-onset seizures showed small reductions (15% to 20% lower) in lacosamide plasma concentrations when lacosamide was coadministered with carbamazepine, phenobarbital or phenytoin.
- Effect of Lacosamide on concomitant AEDs
- Drug-drug interaction studies with other drugs
- Digoxin
There was no effect of lacosamide (400 mg/day) on the pharmacokinetics of digoxin (0.5 mg once daily) in a study in healthy subjects. - Metformin
There were no clinically relevant changes in metformin levels following coadministration of lacosamide (400 mg/day).
Metformin (500 mg three times a day) had no effect on the pharmacokinetics of lacosamide (400 mg/day). - Omeprazole
Omeprazole is a CYP2C19 substrate and inhibitor.
There was no effect of lacosamide (600 mg/day) on the pharmacokinetics of omeprazole (40 mg single dose) in healthy subjects. The data indicated that lacosamide had little in vivo inhibitory or inducing effect on CYP2C19.
Omeprazole at a dose of 40 mg once daily had no effect on the pharmacokinetics of lacosamide (300 mg single dose). However, plasma levels of the O-desmethyl metabolite were reduced about 60% in the presence of omeprazole. - Midazolam
Midazolam is a 3A4 substrate.
There was no effect of lacosamide (200 mg single dose or repeat doses of 400 mg/day given as 200 mg BID) on the pharmacokinetics of midazolam (single dose, 7.5 mg), indicating no inhibitory or inducing effects on CYP3A4. - Oral Contraceptives
There was no influence of lacosamide(400 mg/day) on the pharmacodynamics and pharmacokinetics of an oral contraceptive containing 0.03 mg ethinylestradiol and 0.15 mg levonorgestrel in healthy subjects, except that a 20% increase in ethinylestradiol C max was observed. - Warfarin
Co-administration of lacosamide (400 mg/day) with warfarin (25 mg single dose) did not result in a clinically relevant change in the pharmacokinetic and pharmacodynamic effects of warfarin in a study in healthy male subjects.
- Digoxin
- 100 mg are yellow opaque cap with “A” printed in black ink and a white opaque body with “100” printed in black ink. They are supplied as follows:
- 150 mg are brown opaque cap with “A” printed in black ink and a white opaque body with “150” printed in black ink. They are supplied as follows:
- 200 mg are blue opaque cap with “A” printed in black ink and a white opaque body with “200” printed in black ink. They are supplied as follows:
PR Interval Prolongation, Atrioventricular Block, and Ventricular Tachyarrhythmia
Dose-dependent prolongations in PR interval have been observed in clinical studies of lacosamide, the active moiety in MOTPOLY XR, in adult patients and in healthy volunteers [ see Clinical Pharmacology (12.2)] . In adjunctive clinical trials in adult patients with partial-onset seizures, asymptomatic first-degree atrioventricular (AV) block was observed as an adverse reaction in 0.4% (4/944) of patients randomized to receive lacosamide and 0% (0/364) of patients randomized to receive placebo. One case of profound bradycardia was observed in a patient during a 15-minute infusion of 150 mg lacosamide. When MOTPOLY XR is given with other drugs that prolong the PR interval, further PR prolongation is possible.
In the postmarketing setting, there have been reports of cardiac arrhythmias in patients treated with lacosamide, including bradycardia, AV block, and ventricular tachyarrhythmia, which have rarely resulted in asystole, cardiac arrest, and death. Most, although not all, cases have occurred in patients with underlying proarrhythmic conditions, or in those taking concomitant medications that affect cardiac conduction or prolong the PR interval. These events have occurred with both oral and intravenous routes of administration and at prescribed doses as well as in the setting of overdose [ see Overdosage (10)] .
MOTPOLY XR should be used with caution in patients with underlying proarrhythmic conditions such as known cardiac conduction problems (e.g., marked first-degree AV block, second-degree or higher AV block and sick sinus syndrome without pacemaker), severe cardiac disease (e.g., myocardial ischemia or heart failure, or structural heart disease), and cardiac sodium channelopathies (e.g., Brugada Syndrome). MOTPOLY XR should also be used with caution in patients on concomitant medications that affect cardiac conduction, including sodium channel blockers, beta-blockers, calcium channel blockers, potassium channel blockers, and medications that prolong the PR interval [s ee Drug Interactions (7.2)] . In such patients, obtaining an ECG before beginning MOTPOLY XR, and after MOTPOLY XR is titrated to steady-state maintenance dose, is recommended.
Atrial Fibrillation and Atrial Flutter
In the short-term investigational trials of lacosamide in adult patients with partial-onset seizures there were no cases of atrial fibrillation or flutter. Both atrial fibrillation and atrial flutter have been reported in open label partial-onset seizure trials and in postmarketing experience. In adult patients with diabetic neuropathy, for which lacosamide is not indicated, 0.5% of patients treated with lacosamide experienced an adverse reaction of atrial fibrillation or atrial flutter, compared to 0% of placebo-treated patients. MOTPOLY XR administration may predispose to atrial arrhythmias (atrial fibrillation or flutter), especially in patients with diabetic neuropathy and/or cardiovascular disease.
The studies described below were conducted with immediate-release lacosamide tablets; adverse reactions with MOTPOLY XR are expected to be similar to adverse reactions with immediate-release lacosamide.
Lacosamide in Adults
In the premarketing development of adjunctive therapy for partial-onset seizures, 1327 adult patients received lacosamide in controlled and uncontrolled trials, of whom 1000 were treated for longer than 6 months, and 852 for longer than 12 months. The monotherapy development program for partial-onset seizures included 425 adult patients, 310 of whom were treated for longer than 6 months, and 254 for longer than 12 months.
Partial-Onset Seizures
Monotherapy Historical-Control Trial (Study 1)
In the monotherapy trial for partial-onset seizures, 16% of patients randomized to receive lacosamide at the recommended doses of 300 and 400 mg/day discontinued from the trial as a result of an adverse reaction. The adverse reaction most commonly (≥1% on lacosamide) leading to discontinuation was dizziness.
Adverse reactions that occurred in this study were generally similar to those that occurred in adjunctive placebo-controlled studies. One adverse reaction, insomnia, occurred at a rate of ≥2% and was not reported at a similar rate in previous studies. This adverse reaction has also been observed in postmarketing experience [ see Adverse Reactions (6.2)] . Because this study did not include a placebo control group, causality could not be established.
Dizziness, headache, nausea, somnolence, and fatigue all occurred at lower incidences during the AED Withdrawal Phase and Monotherapy Phase, compared with the Titration Phase [ see Clinical Studies (14.1)] .
Adjunctive Therapy Controlled Trials (Studies 2, 3, and 4)
In adjunctive therapy controlled clinical trials for partial-onset seizures, the rate of discontinuation as a result of an adverse reaction was 8% and 17% in patients randomized to receive lacosamide at the recommended doses of 200 and 400 mg/day, respectively, 29% at 600 mg/day (1.5 times greater than the maximum recommended dose), and 5% in patients randomized to receive placebo. The adverse reactions most commonly (>1% on lacosamide and greater than placebo) leading to discontinuation were dizziness, ataxia, vomiting, diplopia, nausea, vertigo, and blurred vision.
Table 3 gives the incidence of adverse reactions that occurred in ≥2% of adult patients with partial-onset seizures in the lacosamide total group and for which the incidence was greater than placebo.
| Adverse Reaction | Placebo
N=364 % | Lacosamide
200 mg/day N=270 % | Lacosamide
400 mg/day N=471 % | Lacosamide
600 mg/day 600 mg dose is 1.5 times greater than the maximum recommended dose. N=203 % | Lacosamide
Total N=944 % |
|---|---|---|---|---|---|
| Ear and labyrinth disorder | |||||
| Vertigo | 1 | 5 | 3 | 4 | 4 |
| Eye disorders | |||||
| Diplopia | 2 | 6 | 10 | 16 | 11 |
| Blurred Vision | 3 | 2 | 9 | 16 | 8 |
| Gastrointestinal disorders | |||||
| Nausea | 4 | 7 | 11 | 17 | 11 |
| Vomiting | 3 | 6 | 9 | 16 | 9 |
| Diarrhea | 3 | 3 | 5 | 4 | 4 |
| General disorders and administration site conditions | |||||
| Fatigue | 6 | 7 | 7 | 15 | 9 |
| Gait disturbance | <1 | <1 | 2 | 4 | 2 |
| Asthenia | 1 | 2 | 2 | 4 | 2 |
| Injury, poisoning and procedural complications | |||||
| Contusion | 3 | 3 | 4 | 2 | 3 |
| Skin laceration | 2 | 2 | 3 | 3 | 3 |
| Nervous system disorders | |||||
| Dizziness | 8 | 16 | 30 | 53 | 31 |
| Headache | 9 | 11 | 14 | 12 | 13 |
| Ataxia | 2 | 4 | 7 | 15 | 8 |
| Somnolence | 5 | 5 | 8 | 8 | 7 |
| Tremor | 4 | 4 | 6 | 12 | 7 |
| Nystagmus | 4 | 2 | 5 | 10 | 5 |
| Balance disorder | 0 | 1 | 5 | 6 | 4 |
| Memory impairment | 2 | 1 | 2 | 6 | 2 |
| Psychiatric disorders | |||||
| Depression | 1 | 2 | 2 | 2 | 2 |
| Skin and subcutaneous disorders | |||||
| Pruritus | 1 | 3 | 2 | 3 | 2 |
The overall adverse reaction rate was similar in male and female patients. Although there were few non-Caucasian patients, no differences in the incidences of adverse reactions compared to Caucasian patients were observed.
MOTPOLY XR Capsule in Pediatric Patients
Safety of lacosamide was evaluated in clinical studies of pediatric patients for the treatment of partial-onset seizures. Across studies in pediatric patients with partial-onset seizures, 328 patients received lacosamide, of whom 148 received lacosamide for at least 1 year. Adverse reactions reported in clinical studies of pediatric patients were similar to those seen in adult patients.
Laboratory Abnormalities
Abnormalities in liver function tests have occurred in controlled trials with lacosamide in adult patients with partial-onset seizures who were taking 1 to 3 concomitant anti-epileptic drugs. Elevations of ALT to ≥3x ULN occurred in 0.7% (7/935) of lacosamide patients and 0% (0/356) of placebo patients. One case of hepatitis with transaminases >20x ULN occurred in one healthy subject 10 days after lacosamide treatment completion, along with nephritis (proteinuria and urine casts). Serologic studies were negative for viral hepatitis. Transaminases returned to normal within one month without specific treatment. At the time of this event, bilirubin was normal. The hepatitis/nephritis was interpreted as a delayed hypersensitivity reaction to lacosamide.
Other Adverse Reactions
The following is a list of adverse reactions reported by patients treated with lacosamide in all clinical trials in adult patients, including controlled trials and long-term open-label extension trials. Adverse reactions addressed in other tables or sections are not listed here.
Blood and lymphatic system disorders: neutropenia, anemia
Cardiac disorders: palpitations
Ear and labyrinth disorders: tinnitus
Gastrointestinal disorders: constipation, dyspepsia, dry mouth, oral hypoaesthesia
General disorders and administration site conditions: irritability, pyrexia, feeling drunk
Injury, poisoning, and procedural complications: fall
Musculoskeletal and connective tissue disorders: muscle spasms
Nervous system disorders: paresthesia, cognitive disorder, hypoaesthesia, dysarthria, disturbance in attention, cerebellar syndrome
Psychiatric disorders: confusional state, mood altered, depressed mood
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as lacosamide, during pregnancy. Encourage women who are taking MOTPOLY XR during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org/.
Risk Summary
Available data from the North American Antiepileptic Drug (NAAED) pregnancy registry, a prospective cohort study, case reports, and a case series with lacosamide use in pregnant women are insufficient to identify a drug associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. There are no adequate data on the developmental risks associated with the use of MOTPOLY XR in pregnant women. Lacosamide produced developmental toxicity (increased embryofetal and perinatal mortality, growth deficit) in rats following administration during pregnancy. Developmental neurotoxicity was observed in rats following administration during a period of postnatal development corresponding to the third trimester of human pregnancy. These effects were observed at doses associated with clinically relevant plasma exposures ( see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Oral administration of lacosamide to pregnant rats (20, 75, or 200 mg/kg/day) and rabbits (6.25, 12.5, or 25 mg/kg/day) during the period of organogenesis did not produce any effects on the incidences of fetal structural abnormalities. However, the maximum doses evaluated were limited by maternal toxicity in both species and embryofetal death in rats. These doses were associated with maternal plasma lacosamide exposures (AUC) approximately 2 and 1 times (rat and rabbit, respectively) that in humans at the maximum recommended human dose (MRHD) of 400 mg/day.
In two studies in which lacosamide (25, 70, or 200 mg/kg/day and 50, 100, or 200 mg/kg/day) was orally administered to rats throughout pregnancy and lactation, increased perinatal mortality and decreased body weights in the offspring were observed at the highest dose tested. The no-effect dose for pre- and postnatal developmental toxicity in rats (70 mg/kg/day) was associated with a maternal plasma lacosamide AUC similar to that in humans at the MRHD.
Oral administration of lacosamide (30, 90, or 180 mg/kg/day) to rats during the neonatal and juvenile periods of development resulted in decreased brain weights and long-term neurobehavioral changes (altered open field performance, deficits in learning and memory). The early postnatal period in rats is generally thought to correspond to late pregnancy in humans in terms of brain development. The no-effect dose for developmental neurotoxicity in rats was associated with a plasma lacosamide AUC less than that in humans at the MRHD.
In Vitro Data
Lacosamide has been shown in vitro to interfere with the activity of collapsin response mediator protein-2 (CRMP-2), a protein involved in neuronal differentiation and control of axonal outgrowth. Potential adverse effects on CNS development related to this activity cannot be ruled out.
Risk Summary
Data from published literature indicate that lacosamide is present in human milk. There are reports of increased sleepiness in breastfed infants exposed to lacosamide ( see Clinical Considerations). There is no information on the effects of lacosamide on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for MOTPOLY XR and any potential adverse effects on the breastfed infant from MOTPOLY XR or from the underlying maternal condition.
Clinical Considerations
Monitor infants exposed to lacosamide through breastmilk for excess sedation.
The safety and effectiveness of MOTPOLY XR for the treatment of partial-onset seizures have been established in pediatric patients weighing at least 50 kg. Use of MOTPOLY XR in this pediatric group is supported by evidence from adequate and well-controlled studies of lacosamide in adults with partial-onset seizures, pharmacokinetic data from adult and pediatric patients, and safety data in 328 pediatric patients [ see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)and Clinical Studies (14.1, 14.2) ].
Safety and effectiveness of MOTPOLY XR in pediatric patients weighing less than 50 kg have not been established.
MOTPOLY XR extended-release capsules for oral administration contain lacosamide and the following inactive ingredients: ethylcellulose, hypromellose, microcrystalline cellulose, povidone, titanium dioxide, triacetin, triethyl citrate and dye pigments as specified below:
The capsule shells contain the following coloring agents:
100 mg capsules: red iron oxide
150 mg capsules: red iron oxide, yellow iron oxide
200 mg capsules: black iron oxide, FD&C Blue #1, red iron oxide
Cardiac Electrophysiology
Electrocardiographic effects of lacosamide were determined in a double-blind, randomized clinical pharmacology trial of 247 healthy subjects. Chronic oral doses of 400 and 800 mg/day(equal to and two times the maximum recommended dose, respectively) were compared with placebo and a positive control (400 mg moxifloxacin). Lacosamide did not prolong QTc interval and did not have a dose-related or clinically important effect on QRS duration. Lacosamide produced a small, dose-related increase in mean PR interval. At steady-state, the time of the maximum observed mean PR interval corresponded with t max. The placebo-subtracted maximum increase in PR interval (at t max) was 7.3 ms for the 400 mg/day group and 11.9 ms for the 800 mg/day group. For patients who participated in the controlled trials, the placebo-subtracted mean maximum increase in PR interval for a 400 mg/day lacosamide dose was 3.1 ms in patients with partial-onset seizures and 9.4 ms for patients with diabetic neuropathy.
Distribution
The volume of distribution is approximately 0.67 L/kg, which is close to the volume of total body water. Lacosamide is less than 15% bound to plasma proteins.
Metabolism and Elimination
Lacosamide is primarily eliminated from the systemic circulation by renal excretion and biotransformation.
After oral and intravenous administration of 100 mg [14C]-lacosamide approximately 95% of radioactivity administered was recovered in the urine and less than 0.5% in the feces. The major compounds excreted were unchanged lacosamide (approximately 40% of the dose), its O-desmethyl metabolite (approximately 30%), and a structurally unknown polar fraction (~20%). The plasma exposure of the major human metabolite, O-desmethyl-lacosamide, is approximately 10% of that of lacosamide. This metabolite has no known pharmacological activity.
The CYP isoforms mainly responsible for the formation of the major metabolite (O-desmethyl) are CYP3A4, CYP2C9, and CYP2C19. The elimination half-life of the unchanged drug is approximately 13 hours and is not altered by different doses, multiple dosing or intravenous administration.
There is no enantiomeric interconversion of lacosamide.
Specific Populations
Renal Impairment
Lacosamide and its major metabolite are eliminated from the systemic circulation primarily by renal excretion.
The AUC of lacosamide was increased approximately 25% in mildly (CL CR 50-80 mL/min) and moderately (CL CR 30-50 mL/min) and 60% in severely (CL CR≤30 mL/min) renally impaired patients compared to subjects with normal renal function (CL CR>80 mL/min), whereas C max was unaffected. Lacosamide is effectively removed from plasma by hemodialysis. Following a 4-hour hemodialysis treatment, AUC of lacosamide is reduced by approximately 50% [see Dosage and Administration (2.3)] .
Hepatic Impairment
Lacosamide undergoes metabolism. Subjects with moderate hepatic impairment (Child-Pugh B) showed higher plasma concentrations of lacosamide (approximately 50-60% higher AUC compared to healthy subjects). The pharmacokinetics of lacosamide have not been evaluated in severe hepatic impairment [see Dosage and Administration (2.4)] .
Pediatric Patients
The pediatric pharmacokinetic profile of lacosamide was determined in a population pharmacokinetic analysis using sparse plasma concentration data obtained in two open-label studies in 79 pediatric patients with partial-onset seizures that included patients weighing at least 50 kg. The recommended dosing regimen of MOTPOLY XR in pediatric patients weighing at least 50 kg is necessary to achieve lacosamide exposures
similar to those observed in adults treated at effective doses of lacosamide [
see Dosage and Administration (2.1)].
The pharmacokinetics of lacosamide in pediatric patients are similar when used as monotherapy or as adjunctive therapy for the treatment of partial-onset seizures.
Geriatric Patients
In the elderly (>65 years), dose and body-weight normalized AUC and C max is about 20% increased compared to young subjects (18-64 years). This may be related to body weight and decreased renal function in elderly subjects.
Gender
Lacosamide clinical trials indicate that gender does not have a clinically relevant influence on the pharmacokinetics of lacosamide.
Race
There are no clinically relevant differences in the pharmacokinetics of lacosamide between Asian, Black, and Caucasian subjects.
CYP2C19 Polymorphism
There are no clinically relevant differences in the pharmacokinetics of lacosamide between CYP2C19 poor metabolizers and extensive metabolizers [ see Pharmacogenomics (12.5) ].
Drug Interactions
In Vitro Assessment of Drug Interactions
In vitro metabolism studies indicate that lacosamide does not induce the enzyme activity of drug metabolizing cytochrome P450 isoforms CYP1A2, 2B6, 2C9, 2C19 and 3A4. Lacosamide did not inhibit CYP 1A1, 1A2, 2A6, 2B6, 2C8, 2C9, 2D6, 2E1, 3A4/5 at plasma concentrations observed in clinical studies.
In vitro data suggest that lacosamide has the potential to inhibit CYP2C19 at therapeutic concentrations. However, an in vivo study with omeprazole did not show an inhibitory effect on omeprazole pharmacokinetics.
Lacosamide was not a substrate or inhibitor for P-glycoprotein.
Lacosamide is a substrate of CYP3A4, CYP2C9, and CYP2C19. Patients with renal or hepatic impairment who are taking strong inhibitors of CYP3A4 and CYP2C9 may have increased exposure to lacosamide.
Since <15% of lacosamide is bound to plasma proteins, a clinically relevant interaction with other drugs through competition for protein binding sites is unlikely.
In Vivo Assessment of Drug Interactions
Carcinogenesis
There was no evidence of drug related carcinogenicity in mice or rats. Mice and rats received lacosamide once daily by oral administration for 104 weeks at doses producing plasma exposures (AUC) up to approximately 1 and 3 times, respectively, the plasma AUC in humans at the maximum recommended human dose (MRHD) of 400 mg/day.
Mutagenesis
Lacosamide was negative in an in vitro Ames test and an in vivo mouse micronucleus assay. Lacosamide induced a positive response in the in vitro mouse lymphoma assay.
Fertility
No adverse effects on male or female fertility or reproduction were observed in rats at doses producing plasma exposures (AUC) up to approximately 2 times the plasma AUC in humans at the MRHD.
The efficacy of MOTPOLY XR is based on the relative bioavailability of MOTPOLY XR compared to immediate release lacosamide in healthy adults [ see Clinical Pharmacology (12.3)] .
MOTPOLY XR extended-release capsules contain white or off-white beads and are available in the following strengths:
Bottles of 60 NDC 73289-0063-2
Bottles of 60 NDC 73289-0064-2
Bottles of 60 NDC 73289-0065-2
Administration
Inform patients that MOTPOLY XR should be swallowed whole with liquid. Instruct patients to not open, chew, or crush the capsules [ see Dosage and Administration (2.5)] .
Suicidal Thinking and Behavior
Patients, their caregivers, and families should be counseled that AEDs, including MOTPOLY XR, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers [ see Warnings and Precautions (5.1)] .
Dizziness and Ataxia
Patients should be counseled that MOTPOLY XR use may cause dizziness, double vision, abnormal coordination and balance, and somnolence. Patients taking MOTPOLY XR should be advised not to drive, operate complex machinery, or engage in other hazardous activities until they have become accustomed to any such effects associated with MOTPOLY XR [ see Warnings and Precautions (5.2)] .
Cardiac Rhythm and Conduction Abnormalities
Patients should be counseled that MOTPOLY XR is associated with electrocardiographic changes that may predispose to irregular heart beat and syncope. Cardiac arrest has been reported. This risk is increased in patients with underlying cardiovascular disease, with heart conduction problems, or who are taking other medications that affect the heart. Patients should be made aware of and report cardiac signs or symptoms to their healthcare provider right away. Patients who develop syncope should lay down with raised legs and contact their health care provider [ see Warnings and Precautions (5.3)] .
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multi-Organ Hypersensitivity
Patients should be aware that MOTPOLY XR may cause serious hypersensitivity reactions affecting multiple organs such as the liver and kidney. MOTPOLY XR should be discontinued if a serious hypersensitivity reaction is suspected. Patients should also be instructed to report promptly to their physicians any symptoms of liver toxicity (e.g., fatigue, jaundice, dark urine) [ see Warnings and Precautions (5.6)] .
Pregnancy Registry
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during MOTPOLY XR therapy. Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry if they become pregnant. This registry is collecting information about the safety of AEDs during pregnancy [ see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding women using MOTPOLY XR to monitor infants for excess sleepiness and to seek medical care if they notice this sign [
see Use in Specific Populations (8.2)
].
Manufactured for: Aucta Pharmaceuticals, Inc. Piscataway, NJ 08854
LB5040-00
2.4 Dosage Information For Patients With Hepatic Impairment
For patients with mild or moderate hepatic impairment, the maximum recommended dosage is 300 mg. The dose initiation and titration should be based on clinical response and tolerability in patients with hepatic impairment. MOTPOLY XR use is not recommended in patients with severe hepatic impairment.
2.5 Administration Instructions For Motpoly Xr Capsules
MOTPOLY XR may be taken with or without food.
MOTPOLY XR capsules should be swallowed whole with liquid. Do not open, chew, or crush the capsules.
2.6 Discontinuation Of Motpoly Xr
When discontinuing MOTPOLY XR, a gradual withdrawal over at least 1 week is recommended[see Warnings and Precautions (5.5)] .
4 Contraindications
None .
5.1 Suicidal Behavior And Ideation
Antiepileptic drugs (AEDs), including MOTPOLY XR, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number of events is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 2 shows absolute and relative risk by indication for all evaluated AEDs.
| Indication | Placebo Patients with Events Per 1000 Patients | Drug Patients with Events Per 1000 Patients | Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients | Risk Difference: Additional Drug Patients with Events Per 1000 Patients |
|---|---|---|---|---|
| Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
| Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
| Other | 1.0 | 1.8 | 1.9 | 0.9 |
| Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar.
Anyone considering prescribing MOTPOLY XR or any other AED must balance this risk with the risk of untreated illness. Epilepsy and many other illnesses for which antiepileptics are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
5.2 Dizziness And Ataxia
MOTPOLY XR may cause dizziness and ataxia in adult and pediatric patients. In adult patients with partial-onset seizures taking 1 to 3 concomitant AEDs, dizziness was experienced by 25% of patients randomized to the recommended doses (200 to 400 mg/day) of lacosamide (compared with 8% of placebo patients) and was the adverse reaction most frequently leading to discontinuation (3%). Ataxia was experienced by 6% of patients randomized to the recommended doses (200 to 400 mg/day) of lacosamide (compared to 2% of placebo patients). The onset of dizziness and ataxia was most commonly observed during titration. There was a substantial increase in these adverse events at doses higher than 400 mg/day [ see Adverse Reactions (6.1)] .
5.4 Syncope
In the short-term controlled trials of lacosamide in adult patients with partial-onset seizures with no significant system illnesses, there was no increase in syncope compared to placebo. In the short-term controlled trials in adult patients with diabetic neuropathy, for which lacosamide is not indicated, 1.2% of patients who were treated with lacosamide reported an adverse reaction of syncope or loss of consciousness, compared with 0% of placebo-treated patients with diabetic neuropathy. Most of the cases of syncope were observed in patients receiving doses above 400 mg/day. The cause of syncope was not determined in most cases. However, several were associated with either changes in orthostatic blood pressure, atrial flutter/fibrillation (and associated tachycardia), or bradycardia. Cases of syncope have also been observed in open-label clinical partial-onset seizure studies in adult and pediatric patients. These cases were associated with a history of risk factors for cardiac disease and the use of drugs that slow AV conduction.
5.5 Withdrawal Of Antiepileptic Drugs (Aeds)
As with all AEDs, MOTPOLY XR should be withdrawn gradually (over a minimum of 1 week) to minimize the potential of increased seizure frequency in patients with seizure disorders.
5.6 Drug Reaction With Eosinophilia And Systemic Symptoms (Dress)/Multi-Organ Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multi-organ hypersensitivity, has been reported in patients taking antiepileptic drugs, including including lacosamide, the active moiety in MOTPOLY XR. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. This disorder is variable in its expression, and other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity (e.g., fever, lymphadenopathy) may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. MOTPOLY XR should be discontinued if an alternative etiology for the signs or symptoms cannot be established.
6 Adverse Reactions
The following serious adverse reactions are described below and elsewhere in the labeling:
- Suicidal Behavior and Ideation [ see Warnings and Precautions (5.1)]
- Dizziness and Ataxia [ see Warnings and Precautions (5.2)]
- Cardiac Rhythm and Conduction Abnormalities [ see Warnings and Precautions (5.3)]
- Syncope [ see Warnings and Precautions (5.4)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Reactions [ see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of lacosamide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Agranulocytosis
Psychiatric disorders: Aggression, agitation, hallucination, insomnia, psychotic disorder
Skin and subcutaneous tissue disorders: Angioedema, rash, urticaria, Stevens-Johnson syndrome, toxic epidermal necrolysis
Neurologic disorders: Dyskinesia, new or worsening seizures
7.1 Strong Cyp3a4 Or Cyp2c9 Inhibitors
Patients with renal or hepatic impairment who are taking strong inhibitors of CYP3A4 and CYP2C9 may have a significant increase in exposure to MOTPOLY XR. Dose reduction may be necessary in these patients.
7.2 Concomitant Medications That Affect Cardiac Conduction
MOTPOLY XR should be used with caution in patients on concomitant medications that affect cardiac conduction (sodium channel blockers, beta-blockers, calcium channel blockers, potassium channel blockers) including those that prolong PR interval (including sodium channel blocking AEDs), because of a risk of AV block, bradycardia, or ventricular tachyarrhythmia. In such patients, obtaining an ECG before beginning MOTPOLY XR, and after MOTPOLY XR is titrated to steady-state, is recommended.
7.3 Cns Depressants
Concomitant administration of lacosamide and alcohol or other CNS depressant drugs has not been evaluated in clinical studies. Because of the potential of MOTPOLY XR to cause CNS depression, as well as other cognitive and/or neuropsychiatric adverse reactions, MOTPOLY XR should be used with extreme caution if used in combination with alcohol and other CNS depressants.
8.5 Geriatric Use
There were insufficient numbers of elderly patients enrolled in partial-onset seizure trials (n=18) to adequately determine whether they respond differently from younger patients.
No MOTPOLY XR dose adjustment based on age is necessary. In elderly patients, dose titration should be performed with caution, usually starting at the lower end of the dosing range, reflecting the greater frequency of decreased hepatic function, decreased renal function, increased cardiac conduction abnormalities, and polypharmacy [see Dosage and Administration (2.1, 2.3, 2.4) and Clinical Pharmacology (12.3)] .
8.6 Renal Impairment
No dose adjustment is necessary in patients with mild to moderate renal impairment (CL CR ≥30 mL/min). In patients with severe renal impairment [creatinine clearance (CL CR) less than 30 mL/min as estimated by the Cockcroft-Gault equation for adults; CL CR less than 30 mL/min/1.73m 2 as estimated by the Schwartz equation for pediatric patients ] and in those with end-stage renal disease, the maximum recommended dosage is 300 mg once daily [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)] .
In all patients with renal impairment, dose initiation and titration should be based on clinical response and tolerability.
MOTPOLY XR is effectively removed from plasma by hemodialysis. Dosage supplementation of up to 50% following hemodialysis should be considered.
8.7 Hepatic Impairment
For adult and pediatric patients with mild to moderate hepatic impairment, the maximum recommended dosage is 300 mg once daily. Patients with mild to moderate hepatic impairment should be observed closely for adverse reactions, and dose initiation and titration should be based on clinical response and tolerability [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)] .
The pharmacokinetics of lacosamide has not been evaluated in severe hepatic impairment. MOTPOLY XR use is not recommended in patients with severe hepatic impairment.
9.1 Controlled Substance
MOTPOLY XR contains lacosamide, a Schedule V controlled substance.
9.2 Abuse
Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. In a human abuse potential study, single doses of 200 mg and 800 mg lacosamide (equal to and 2 times the recommended maintenance dosage, respectively) produced euphoria-type subjective responses that differentiated statistically from placebo; at 800 mg, these euphoria-type responses were statistically indistinguishable from those produced by alprazolam, a Schedule IV drug. The duration of the euphoria-type responses following lacosamide was less than that following alprazolam. A high rate of euphoria was also reported as an adverse event in the human abuse potential study following single doses of 800 mg lacosamide (15% [5/34]) compared to placebo (0%) and in two pharmacokinetic studies following single and multiple doses of 300-800 mg lacosamide (ranging from 6% [2/33] to 25% [3/12]) compared to placebo (0%). However, the rate of euphoria reported as an adverse event in the lacosamide development program at therapeutic doses was less than 1%.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt termination of lacosamide in clinical trials with diabetic neuropathic pain patients produced no signs or symptoms that are associated with a withdrawal syndrome indicative of physical dependence. However, psychological dependence cannot be excluded due to the ability of lacosamide to produce euphoria-type adverse events in humans.
10 Overdosage
Events reported after an intake of more than 800 mg (twice the maximum recommended daily dosage) of lacosamide include dizziness, nausea, and seizures (generalized tonic-clonic seizures, status epilepticus). Cardiac conduction disorders, confusion, decreased level of consciousness, cardiogenic shock, cardiac arrest, and coma have also been observed. Fatalities have occurred following lacosamide overdoses of several grams.
There is no specific antidote for overdose with lacosamide. Standard decontamination procedures should be followed. General supportive care of the patient is indicated including monitoring of vital signs and observation of the clinical status of patient. A Certified Poison Control Center should be contacted for up to date information on the management of overdose with lacosamide.
Standard hemodialysis procedures result in significant clearance of lacosamide (reduction of systemic exposure by 50% in 4 hours). Hemodialysis may be indicated based on the patient's clinical state or in patients with significant renal impairment.
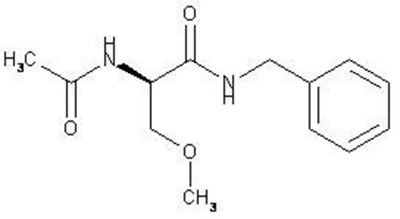
11 Description
The chemical name of lacosamide, the single (R)-enantiomer, is (R)-2-acetamido-N-benzyl-3-methoxypropionamide (IUPAC). Lacosamide is a functionalized amino acid. Its molecular formula is C 13H 18N 2O 3 and its molecular weight is 250.30. The chemical structure is:
Lacosamide is a white to light yellow powder. It is sparingly soluble in water and slightly soluble in acetonitrile and ethanol.
12.1 Mechanism Of Action
The precise mechanism by which MOTPOLY XR exerts its antiepileptic effects in humans remains to be fully elucidated. In vitro electrophysiological studies have shown that lacosamide selectively enhances slow inactivation of voltage-gated sodium channels, resulting in stabilization of hyperexcitable neuronal membranes and inhibition of repetitive neuronal firing.
12.2 Pharmacodynamics
A pharmacokinetic-pharmacodynamic (efficacy) analysis was performed based on the pooled data from the 3 efficacy trials for partial-onset seizures. Lacosamide exposure is correlated with the reduction in seizure frequency. However, doses above 400 mg/day do not appear to confer additional benefit in group analyses.
12.3 Pharmacokinetics
Absorption
Pharmacokinetic information for MOTPOLY XR following oral administration was obtained from studies conducted in healthy adult subjects.
Lacosamide is completely absorbed after oral administration with negligible first-pass effect with a high absolute bioavailability of approximately 100%. In a multiple-dose pharmacokinetic study in healthy adult subjects, at steady-state, the peak plasma lacosamide concentrations (T max) after oral administration of MOTPOLY XR was reached in 7 hours. Steady state plasma concentrations are achieved after 4 days of once daily repeated administration. Pharmacokinetics of lacosamide are dose proportional (100-800 mg).
Effect of Food
Compared to the fasted state, high-fat meal has no effect on the C max, T max and AUC of MOTPOLY XR. Hence, MOTPOLY XR can be taken with or without food
12.5 Pharmacogenomics
Results from a trial in poor metabolizers (PM) (N=4) and extensive metabolizers (EM) (N=8) of cytochrome P450 (CYP) 2C19 showed that lacosamide plasma concentrations were similar in PMs and EMs, but plasma concentrations and the amount excreted into urine of the O-desmethyl metabolite were about 70% reduced in PMs compared to EMs. This difference is not clinically significant [ see Clinical Pharmacology (12.3)] .
14.1 Monotherapy In Patients With Partial-Onset Seizures
The efficacy of immediate-release lacosamide in monotherapy was established in a historical-control, multicenter, randomized trial that included 425 patients, age 16 to 70 years, with partial-onset seizures (Study 1). To be included in Study 1, patients were required to be taking stable doses of 1 or 2 marketed antiepileptic drugs. This treatment continued into the 8-week baseline period. To remain in the study, patients were required to have at least 2 partial-onset seizures per 28 days during the 8-week baseline period. The baseline period was followed by a 3-week titration period, during which lacosamide was added to the ongoing antiepileptic regimen. This was followed by a 16-week maintenance period (i.e., a 6-week withdrawal period for background antiepileptic drugs, followed by a 10-week monotherapy period). Patients were randomized 3 to 1 to receive lacosamide 400 mg/day or lacosamide 300 mg/day. Treatment assignments were blinded. Response to treatment was based upon a comparison of the number of patients who met exit criteria during the maintenance phase, compared to historical controls. The historical control consisted of a pooled analysis of the control groups from 8 studies of similar design, which utilized a sub-therapeutic dose of an antiepileptic drug. Statistical superiority to the historical control was considered to be demonstrated if the upper limit from a 2-sided 95% confidence interval for the percentage of patients meeting exit criteria in patients receiving lacosamide remained below the lower 95% prediction limit of 65% derived from the historical control data.
The exit criteria were one or more of the following: (1) doubling of average monthly seizure frequency during any 28 consecutive days, (2) doubling of highest consecutive 2-day seizure frequency, (3) clinically significant prolongation or worsening of overall seizure duration, frequency, type or pattern considered by the investigator to require trial discontinuation, (4) status epilepticus or new onset of serial/cluster seizures. The study population profile appeared comparable to that of the historical control population.
For the lacosamide 400 mg/day group, the estimate of the percentage of patients meeting at least 1 exit criterion was 30% (95% CI: 25%, 36%). The upper limit of the 2-sided 95% CI (36%) was below the threshold of 65% derived from the historical control data, meeting the pre-specified criteria for efficacy. Lacosamide 300 mg/day also met the pre-specified criteria for efficacy.
14.2 Adjunctive Therapy In Patients With Partial-Onset Seizures
The efficacy of lacosamide as adjunctive therapy in partial-onset seizures was established in three 12-week, randomized, double-blind, placebo-controlled, multicenter trials in adult patients (Study 2, Study 3, and Study 4). Enrolled patients had partial-onset seizures with or without secondary generalization and were not adequately controlled with 1 to 3 concomitant AEDs. During an 8-week baseline period, patients were required to have an average of ≥4 partial-onset seizures per 28 days with no seizure-free period exceeding 21 days. In these 3 trials, patients had a mean duration of epilepsy of 24 years and a median baseline seizure frequency ranging from 10 to 17 per 28 days. 84% of patients were taking 2 to 3 concomitant AEDs with or without concurrent vagal nerve stimulation.
Study 2 compared doses of lacosamide 200, 400, and 600 mg/day with placebo. Study 3 compared doses of lacosamide 400 and 600 mg/day with placebo. Study 4 compared doses of lacosamide 200 and 400 mg/day with placebo. In all three trials, following an 8-week baseline phase to establish baseline seizure frequency prior to randomization, patients were randomized and titrated to the randomized dose (a 1-step back-titration of lacosamide 100 mg/day or placebo was allowed in the case of intolerable adverse events at the end of the titration phase). During the titration phase, in all 3 adjunctive therapy trials, treatment was initiated at 100 mg/day (50 mg twice daily), and increased in weekly increments of 100 mg/day to the target dose. The titration phase lasted 6 weeks in Study 2 and Study 3, and 4 weeks in Study 4. In all three trials, the titration phase was followed by a maintenance phase that lasted 12 weeks, during which patients were to remain on a stable dose of lacosamide.
A reduction in 28-day seizure frequency (baseline to maintenance phase), as compared to the placebo group, was the primary variable in all three adjunctive therapy trials. A statistically significant effect was observed with lacosamide treatment (Figure 1) at doses of 200 mg/day (Study 4), 400 mg/day (Studies 2, 3, and 4), and 600 mg/day (Studies 2 and 3).
Subset evaluations of lacosamide demonstrate no important differences in seizure control as a function of gender or race, although data on race was limited (about 10% of patients were non-Caucasian).
Figure 2 presents the percentage of patients (X-axis) with a percent reduction in partial seizure frequency (responder rate) from baseline to the maintenance phase at least as great as that represented on the Y-axis. A positive value on the Y-axis indicates an improvement from baseline (i.e., a decrease in seizure frequency), while a negative value indicates a worsening from baseline (i.e., an increase in seizure frequency). Thus, in a display of this type, a curve for an effective treatment is shifted to the left of the curve for placebo. The proportion of patients achieving any particular level of reduction in seizure frequency was consistently higher for the lacosamide groups, compared to the placebo group. For example, 40% of patients randomized to lacosamide (400 mg/day) experienced a 50% or greater reduction in seizure frequency, compared to 23% of patients randomized to placebo. Patients with an increase in seizure frequency >100% are represented on the Y-axis as equal to or greater than -100%.
16.2 Storage And Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F).
[See USP Controlled Room Temperature]
17 Patient Counseling Information
Advise the patient or caregiver to read the FDA-approved patient labeling (Medication Guide).
Spl Medguide
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: 05/2022 | ||
MEDICATION GUIDE lacosamide oral use, CV | |||
| Read this Medication Guide before you start taking MOTPOLY XR and each time you get a refill. There may be new information. This Medication Guide describes important safety information about MOTPOLY XR. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. | |||
What is the most important information I should know about MOTPOLY XR?
1.
Like other antiepileptic drugs, MOTPOLY XR may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
| |||
|
| ||
| How can I watch for early symptoms of suicidal thoughts and actions? | |||
| |||
2.MOTPOLY XR may cause you to feel dizzy, have double vision, feel sleepy, or have problems with coordination and walking. Do not drive, operate heavy machinery, or do other dangerous activities until you know how MOTPOLY XR affects you. 3.MOTPOLY XR may cause you to have an irregular heartbeat or may cause you to faint. In rare cases, cardiac arrest has been reported. Call your healthcare provider right away if you: | |||
|
| ||
| If you have fainted or feel like you are going to faint you should lay down with your legs raised. | |||
| 4.MOTPOLY XR is a federally controlled substance (CV) because it can be abused or lead to drug dependence. Keep your MOTPOLY XR in a safe place, to protect it from theft. Never give your MOTPOLY XR to anyone else, because it may harm them. Selling or giving away this medicine is against the law. | |||
What is MOTPOLY XR? MOTPOLY XR is a prescription medicine used to treat partial-onset seizures in adults and in children weighing at least 110 pounds (50 kg). It is not known if MOTPOLY XR is safe and effective for partial-onset seizures in children weighing less than 50 kg. | |||
| What should I tell my healthcare provider before taking MOTPOLY XR?
Before you take MOTPOLY XR, tell your healthcare provider about all of your medical conditions, including if you :
Taking MOTPOLY XR with certain other medicines may cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine. | |||
How should I take MOTPOLY XR?
| |||
What should I avoid while taking MOTPOLY XR?
| |||
What are the possible side effects of MOTPOLY XR?
| |||
|
| ||
| The most common side effects of MOTPOLY XR include: | |||
|
| ||
| These are not all of the possible side effects of MOTPOLY XR. For more information ask your healthcare provider or pharmacist. Tell your healthcare provider about any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
How should I store MOTPOLY XR?
| |||
| General Information about the safe and effective use of MOTPOLY XR.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use MOTPOLY XR for a condition for which it was not prescribed. Do not give MOTPOLY XR to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about MOTPOLY XR that is written for health professionals. | |||
| What are the ingredients in MOTPOLY XR?
Active ingredient : lacosamide Capsule inactive ingredients: ethylcellulose, hypromellose, microcrystalline cellulose, povidone, titanium dioxide, triacetin, triethyl citrate, and additional ingredients listed below:
| |||
Principal Display Panel - 100 Mg Capsule Bottle Label
NDC 73289-0063-2
Motpoly XR (lacosamide) extended-release capsules
CV
100 mg
Rx only 60 Capsules
ATTENTION PHARMACIST:
Each patient is required to receive
the accompanying Medication Guide
Each capsule contains
100 mg lacosamide.
Keep this and all drugs
out of reach of children.
Recommended Dosage:
See prescribing Information.
Swallow capsules whole
with liquid. Do not open,
chew or crush.
Manufactured for:
Aucta Pharmoceutlcals, Inc.
Piscataway, NJ 08854
Store at 20˚ C to 25˚ C (68˚ F to 77˚ F);
excursions permitted between 15˚ C to
30˚ C (59˚ F to 86˚ F).
[See USP Controlled Room Temperature]
Principal Display Panel - 150 Mg Capsule Bottle Label
NDC 73289-0064-2
Motpoly XR (lacosamide) extended-release capsules
CV
150 mg
Rx only
60 Capsules
ATTENTION PHARMACIST:
Each patient is required to receive the
accompanying Medication Guide.
Each capsule contains
150 mg lacosamide.
Keep this and all drugs
out of reach of children.
Recommended Dosage:
See prescribing information.
Swallow capsules whole
with liquid. Do not open,
chew or crush.
Manufactured for:
Aucta Pharmaceuticals, Inc.
Piscataway, NJ 08854
Store at 20˚ C to 25˚ C (68˚ F to 77˚ F);
excursions permitted between 15˚ C to
30˚ C (59˚ F to 86˚ F).
[See USP Controlled Room Temperature]
Principal Display Panel - 200 Mg Capsule Bottle Label
NDC 73289-0065-2
Motpoly XR (lacosamide) extended-release capsules
CV
200 mg
Rx only
60 Capsules
ATTENTION PHARMACIST:
Each patient is required to receive the
accompanying Medication Guide.
Each capsule contains
200 mg lacosamide.
Keep this and all drugs
out of reach of children.
Recommended Dosage:
See prescribing information.
Swallow capsules whole
with liquid. Do not open,
chew or crush.
Manufactured for:
Aucta Pharmaceuticals, Inc.
Piscataway, NJ 08854
Store at 20˚ C to 25˚ C (68˚ F to 77˚ F);
excursions permitted between 15˚ C to
30˚ C (59˚ F to 86˚ F).
[See USP Controlled Room Temperature]
Principal Display Panel - Physician Samples
Professional Sample – Not for Sale
NDC 73289-0063-1
Motpoly XR (lacosamide) extended-release capsules
CV
100 mg per capsule
ATTENTION PHYSICIAN:
Each patient is required to receive
the accompanying Medication Guide.
Rx Only 14 Capsules
Each capsule contains 100 mg lacosamide.
Recommended Dosage: See prescribing information.
Swallow capsules whole with liquid. Do not open, chew or crush.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between
15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature].
Manufactured for:
Aucta Pharmaceuticals, Inc.
Piscataway, NJ 08854
* Please review the disclaimer below.