FDA Label for Lisinopril
View Indications, Usage & Precautions
- WARNING: FETAL TOXICITY
- 1.1 HYPERTENSION
- 1.2 HEART FAILURE
- 1.3 REDUCTION OF MORTALITY IN ACUTE MYOCARDIAL INFARCTION
- 2.1 HYPERTENSION
- 2.2 HEART FAILURE
- 2.3 REDUCTION OF MORTALITY IN ACUTE MYOCARDIAL INFARCTION
- 2.4 DOSE IN PATIENTS WITH RENAL IMPAIRMENT
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5.1 FETAL TOXICITY
- 5.2 ANGIOEDEMA AND ANAPHYLACTOID REACTIONS
- 5.3 IMPAIRED RENAL FUNCTION
- 5.4 HYPOTENSION
- 5.5 HYPERKALEMIA
- 5.6 HEPATIC FAILURE
- 6.1 CLINICAL TRIALS EXPERIENCE
- 6.2 POST-MARKETING EXPERIENCE
- 7.1 DIURETICS
- 7.2 ANTIDIABETICS
- 7.3 NON-STEROIDAL ANTI-INFLAMMATORY AGENTS INCLUDING SELECTIVE CYCLOOXYGENASE-2 INHIBITORS (COX-2 INHIBITORS)
- 7.4 DUAL BLOCKADE OF THE RENIN-ANGIOTENSIN SYSTEM (RAS)
- 7.5 LITHIUM
- 7.6 GOLD
- 7.7 MTOR INHIBITORS
- 8.1 PREGNANCY
- 8.2 LACTATION
- 8.4 PEDIATRIC USE
- 8.5 GERIATRIC USE
- 8.6 RACE
- 8.7 RENAL IMPAIRMENT
- 10 OVERDOSAGE
- 11 DESCRIPTION
- 12.1 MECHANISM OF ACTION
- 12.2 PHARMACODYNAMICS
- 12.3 PHARMACOKINETICS
- 13.1 CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- 14.1 HYPERTENSION
- 14.2 HEART FAILURE
- 14.3 ACUTE MYOCARDIAL INFARCTION
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE/LABEL DISPLAY PANEL
Lisinopril Product Label
The following document was submitted to the FDA by the labeler of this product Exelan Pharmaceuticals Inc.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Warning: Fetal Toxicity
- When pregnancy is detected, discontinue Lisinopril as soon as possible [see Warnings and Precautions (5.1)].
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)].
1.1 Hypertension
Lisinopril tablets are indicated for the treatment of hypertension in adult patients and pediatric patients 6 years of age and older to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Lisinopril may be administered alone or with other antihypertensive agents [see Clinical Studies (14.1)].
1.2 Heart Failure
Lisinopril tablets are indicated to reduce signs and symptoms of systolic heart failure [see Clinical Studies (14.2)].
1.3 Reduction Of Mortality In Acute Myocardial Infarction
Lisinopril tablets are indicated for the reduction of mortality in treatment of hemodynamically stable patients within 24 hours of acute myocardial infarction. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin and beta-blockers [see Clinical Studies (14.3)].
2.1 Hypertension
Initial Therapy in adults: The recommended initial dose is 10 mg once a day. Dosage should be adjusted according to blood pressure response. The usual dosage range is 20 mg to 40 mg per day administered in a single daily dose. Doses up to 80 mg have been used but do not appear to give greater effect.
Use with diuretics in adults
If blood pressure is not controlled with lisinopril alone, a low dose of a diuretic may be added (e.g. hydrochlorothiazide, 12.5 mg). After the addition of a diuretic, it may be possible to reduce the dose of lisinopril.
The recommended starting dose in adult patients with hypertension taking diuretics is 5 mg once per day.
Pediatric Patients 6 years of age and older with hypertension
For pediatric patients with glomerular filtration rate > 30 mL/min/1.73m2, the recommended starting dose is 0.07 mg per kg once daily (up to 5 mg total). Dosage should be adjusted according to blood pressure response up to a maximum of 0.61 mg per kg (up to 40 mg) once daily. Doses above 0.61 mg per kg (or in excess of 40 mg) have not been studied in pediatric patients [see Clinical Pharmacology (12.3)].
Lisinopril is not recommended in pediatric patients < 6 years or in pediatric patients with glomerular filtration rate < 30 mL/min/1.73 m2 [see Use in Specific Populations (8.4) and Clinical Studies (14.1)].
2.2 Heart Failure
The recommended starting dose for lisinopril, when used with diuretics and (usually) digitalis as adjunctive therapy for systolic heart failure, is 5 mg once daily. The recommended starting dose in these patients with hyponatremia (serum sodium < 130 mEq/L) is 2.5 mg once daily. Increase as tolerated to a maximum of 40 mg once daily.
Diuretic dose may need to be adjusted to help minimize hypovolemia, which may contribute to hypotension [see Warnings and Precautions (5.4), and Drug Interactions (7.1)]. The appearance of hypotension after the initial dose of lisinopril does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension.
2.3 Reduction Of Mortality In Acute Myocardial Infarction
In hemodynamically stable patients within 24 hours of the onset of symptoms of acute myocardial infarction, give lisinopril 5 mg orally, followed by 5 mg after 24 hours, 10 mg after 48 hours and then 10 mg once daily. Dosing should continue for at least six weeks.
Initiate therapy with 2.5 mg in patients with a low systolic blood pressure (≤ 120 mmHg and > 100 mmHg) during the first 3 days after the infarct [see Warnings and Precautions (5.4)]. If hypotension occurs (systolic blood pressure ≤ 100 mmHg) a daily maintenance dose of 5 mg may be given with temporary reductions to 2.5 mg if needed. If prolonged hypotension occurs (systolic blood pressure < 90 mmHg for more than 1 hour) lisinopril should be withdrawn.
2.4 Dose In Patients With Renal Impairment
No dose adjustment of lisinopril is required in patients with creatinine clearance > 30 mL/min. In patients with creatinine clearance ≥ 10 mL/min and ≤ 30 mL/min, reduce the initial dose of lisinopril to half of the usual recommended dose i.e., hypertension, 5 mg; systolic heart failure, 2.5 mg and acute MI, 2.5 mg. Up titrate as tolerated to a maximum of 40 mg daily. For patients on hemodialysis or creatinine clearance < 10 mL/min, the recommended initial dose is 2.5 mg once daily [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
3 Dosage Forms And Strengths
2.5 mg Tablets: White, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “417” on the other side.
5 mg Tablets: Red, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “418” on the other side.
10 mg Tablets: Red, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “419” on the other side.
20 mg Tablets: Red, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “420” on the other side.
30 mg Tablets: Red, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “421” on the other side.
40 mg Tablets: Yellow, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “422” on the other side.
4 Contraindications
Lisinopril is contraindicated in patients with:
- a history of angioedema or hypersensitivity related to previous treatment with an angiotensin converting enzyme inhibitor
- hereditary or idiopathic angioedema
Do not co-administer aliskiren with lisinopril in patients with diabetes [see Drug Interactions (7.4)]
5.1 Fetal Toxicity
Lisinopril can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue lisinopril as soon as possible [see Use in specific Populations (8.1)].
5.2 Angioedema And Anaphylactoid Reactions
Patients taking concomitant mTOR inhibitor (e.g. temsirolimus, sirolimus, everolimus) therapy may be at increased risk for angioedema. [see Drug Interactions (7.7)].
Angioedema
Head and Neck Angioedema
Angioedema of the face, extremities, lips, tongue, glottis and/or larynx, including some fatal reactions, have occurred in patients treated with angiotensin converting enzyme inhibitors, including lisinopril, at any time during treatment. Patients with involvement of the tongue, glottis or larynx are likely to experience airway obstruction, especially those with a history of airway surgery. Lisinopril should be promptly discontinued and appropriate therapy and monitoring should be provided until complete and sustained resolution of signs and symptoms of angioedema has occurred.
Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving an ACE inhibitor [see Contraindications (4)]. ACE inhibitors have been associated with a higher rate of angioedema in black than in non-black patients.
Intestinal Angioedema
Intestinal angioedema has occurred in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. In some cases, the angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor.
Anaphylactoid Reactions
Anaphylactoid Reactions During Desensitization
Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions.
Anaphylactoid Reactions During Dialysis
Sudden and potentially life threatening anaphylactoid reactions have occurred in some patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. In such patients, dialysis must be stopped immediately, and aggressive therapy for anaphylactoid reactions must be initiated. Symptoms have not been relieved by antihistamines in these situations. In these patients, consideration should be given to using a different type of dialysis membrane or a different class of antihypertensive agent. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
5.3 Impaired Renal Function
Monitor renal function periodically in patients treated with lisinopril. Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, post-myocardial infarction or volume depletion) may be at particular risk of developing acute renal failure on lisinopril. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on lisinopril [see Adverse Reactions (6.1), Drug Interactions (7.4)].
5.4 Hypotension
Lisinopril can cause symptomatic hypotension, sometimes complicated by oliguria, progressive azotemia, acute renal failure or death. Patients at risk of excessive hypotension include those with the following conditions or characteristics: heart failure with systolic blood pressure below 100 mmHg, ischemic heart disease, cerebrovascular disease, hyponatremia, high dose diuretic therapy, renal dialysis, or severe volume and/or salt depletion of any etiology.
In these patients, lisinopril should be started under very close medical supervision and such patients should be followed closely for the first two weeks of treatment and whenever the dose of lisinopril and/or diuretic is increased. Avoid use of lisinopril in patients who are hemodynamically unstable after acute MI.
Symptomatic hypotension is also possible in patients with severe aortic stenosis or hypertrophic cardiomyopathy.
Surgery/Anesthesia
In patients undergoing major surgery or during anesthesia with agents that produce hypotension, lisinopril may block angiotensin II formation secondary to compensatory renin release. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
5.5 Hyperkalemia
Serum potassium should be monitored periodically in patients receiving lisinopril. Drugs that inhibit the renin angiotensin system can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements and/or potassium-containing salt substitutes [see Drug Interactions (7.1)].
5.6 Hepatic Failure
ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice or hepatitis and progresses to fulminant hepatic necrosis and sometimes death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical treatment.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Hypertension
In clinical trials in patients with hypertension treated with lisinopril, 5.7% of patients on lisinopril discontinued with adverse reactions.
The following adverse reactions (events 2% greater on lisinopril than on placebo) were observed with lisinopril alone: headache (by 3.8%), dizziness (by 3.5%), cough (by 2.5%).
Heart Failure
In patients with systolic heart failure treated with lisinopril for up to four years, 11% discontinued therapy with adverse reactions. In controlled studies in patients with heart failure, therapy was discontinued in 8.1% of patients treated with lisinopril for 12 weeks, compared to 7.7% of patients treated with placebo for 12 weeks.
The following adverse reactions (events 2% greater on lisinopril than on placebo) were observed with lisinopril: hypotension (by 3.8%), chest pain (by 2.1%).
In the two-dose ATLAS trial [see Clinical Studies (14.2)] in heart failure patients, withdrawals due to adverse reactions were not different between the low and high groups, either in total number of discontinuation (17% to 18%) or in rare specific reactions (< 1%). The following adverse reactions, mostly related to ACE inhibition, were reported more commonly in the high dose group:
| High Dose (n=1568) | Low Dose (n=1596) | |
| Dizziness | 19% | 12% |
| Hypotension | 11% | 7% |
| Creatinine increased | 10% | 7% |
| Hyperkalemia | 6% | 4% |
| Syncope | 7% | 5% |
Acute Myocardial Infarction
Patients treated with lisinopril had a higher incidence of hypotension (by 5.3%) and renal dysfunction (by 1.3%) compared with patients not taking lisinopril.
Other clinical adverse reactions occurring in 1% or higher of patients with hypertension or heart failure treated with lisinopril in controlled clinical trials and do not appear in other sections of labeling are listed below:
Body as a whole: Fatigue, asthenia, orthostatic effects.
Digestive: Pancreatitis, constipation, flatulence, dry mouth, diarrhea.
Hematologic: Rare cases of bone marrow depression, hemolytic anemia, leukopenia/neutropenia and thrombocytopenia.
Endocrine: Diabetes mellitus, inappropriate antidiuretic hormone secretion.
Metabolic: Gout.
Skin: Urticaria, alopecia, photosensitivity, erythema, flushing, diaphoresis, cutaneous pseudolymphoma, toxic epidermal necrolysis, Stevens - Johnson syndrome, and pruritus.
Special Senses: Visual loss, diplopia, blurred vision, tinnitus, photophobia, taste disturbances, olfactory disturbance.
Urogenital: Impotence.
Miscellaneous: A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, eosinophilia, leukocytosis, paresthesia and vertigo. Rash, photosensitivity or other dermatological manifestations may occur alone or in combination with these symptoms.
Clinical Laboratory Test Findings
Serum Potassium: In clinical trials hyperkalemia (serum potassium greater than 5.7 mEq/L) occurred in 2.2% and 4.8% of lisinopril-treated patients with hypertension and heart failure, respectively [see Warnings and Precautions (5.5)].
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen and serum creatinine, reversible upon discontinuation of therapy, were observed in about 2% of patients with hypertension treated with lisinopril alone. Increases were more common in patients receiving concomitant diuretics and in patients with renal artery stenosis [see Warnings and Precautions (5.4)]. Reversible minor increases in blood urea nitrogen and serum creatinine were observed in 11.6% of patients with heart failure on concomitant diuretic therapy. Frequently, these abnormalities resolved when the dosage of the diuretic was decreased.
Patients with acute myocardial infarction in the GISSI-3 trial treated with lisinopril had a higher (2.4% versus 1.1% in placebo) incidence of renal dysfunction in-hospital and at six weeks (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration).
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.4 g% and 1.3 vol%, respectively) occurred frequently in patients treated with lisinopril but were rarely of clinical importance in patients without some other cause of anemia. In clinical trials, less than 0.1% of patients discontinued therapy due to anemia.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of lisinopril that are not included in other sections of labeling. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Other reactions include:
Metabolism and nutrition disorders
Hyponatremia [see Warnings and Precautions (5.4)], cases of hypoglycemia in diabetic patients on oral antidiabetic agents or insulin [see Drug Interactions (7.2)]
Nervous system and psychiatric disorders
Mood alterations (including depressive symptoms), mental confusion, hallucinations
Skin and subcutaneous tissue disorders
Psoriasis
7.1 Diuretics
Initiation of lisinopril in patients on diuretics may result in excessive reduction of blood pressure. The possibility of hypotensive effects with lisinopril can be minimized by either decreasing or discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with lisinopril. If this is not possible, reduce the starting dose of lisinopril [see Dosage and Administration (2.2) and Warnings and Precautions (5.4)].
Lisinopril attenuates potassium loss caused by thiazide-type diuretics. Potassium-sparing diuretics (spironolactone, amiloride, triamterene, and others) can increase the risk of hyperkalemia. Therefore, if concomitant use of such agents is indicated, monitor the patient’s serum potassium frequently.
7.2 Antidiabetics
Concomitant administration of lisinopril and antidiabetic medicines (insulins, oral hypoglycemic agents) may cause an increased blood-glucose-lowering effect with risk of hypoglycemia.
7.3 Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (Cox-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including lisinopril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving lisinopril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including lisinopril, may be attenuated by NSAIDs.
7.4 Dual Blockade Of The Renin-Angiotensin System (Ras)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy.
The VA NEPHRON trial enrolled 1448 patients with type 2 diabetes, elevated urinary-albumin-to-creatinine ratio, and decreased estimated glomerular filtration rate (GFR 30 to 89.9 ml/min), randomized them to lisinopril or placebo on a background of losartan therapy and followed them for a median of 2.2 years. Patients receiving the combination of losartan and lisinopril did not obtain any additional benefit compared to monotherapy for the combined endpoint of decline in GFR, end state renal disease, or death, but experienced an increased incidence of hyperkalemia and acute kidney injury compared with the monotherapy group.
In general, avoid combined use of RAS inhibitors. Closely monitor blood pressure, renal function and electrolytes in patients on lisinopril and other agents that affect the RAS.
Do not co-administer aliskiren with lisinopril in patients with diabetes. Avoid use of aliskiren with lisinopril in patients with renal impairment (GFR <60 ml/min).
7.5 Lithium
Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs, which cause elimination of sodium, including ACE inhibitors. Lithium toxicity was usually reversible upon discontinuation of lithium and the ACE inhibitor. Monitor serum lithium levels during concurrent use.
7.6 Gold
Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including Lisinopril.
7.7 Mtor Inhibitors
Patients taking concomitant mTOR inhibitor (e.g. temsirolimus, sirolimus, everolimus) therapy may be at increased risk for angioedema [see Warnings and Precautions (5.2)].
8.1 Pregnancy
Risk Summary
Lisinopril can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. When pregnancy is detected, discontinue lisinopril as soon as possible. The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. In the general U.S. population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section, and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions
Oligohydramnios in pregnant women who use drugs affecting the renin-angiotensin system in the second and third trimesters of pregnancy can result in the following: reduced fetal renal function leading to anuria and renal failure, fetal lung hypoplasia and skeletal deformations, including skull hypoplasia, hypotension, and death. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus.
Perform serial ultrasound examinations to assess the intra-amniotic environment. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to lisinopril for hypotension, oliguria, and hyperkalemia. If oliguria or hypotension occur in neonates with a history of in utero exposure to lisinopril, support blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and substituting for disordered renal function.
8.2 Lactation
No data are available regarding the presence of lisinopril in human milk or the effects of lisinopril on the breast fed infant or on milk production. Lisinopril is present in rat milk. Because of the potential for severe adverse reactions in the breastfed infant, advise women not to breastfeed during treatment with lisinopril.
8.4 Pediatric Use
Antihypertensive effects and safety of lisinopril have been established in pediatric patients aged 6 to 16 years [see Dosage and Administration (2.1) and Clinical Studies (14.1)]. No relevant differences between the adverse reaction profile for pediatric patients and adult patients were identified.
Safety and effectiveness of lisinopril have not been established in pediatric patients under the age 6 or in pediatric patients with glomerular filtration rate < 30 mL/min/1.73 m2 [see Dosage and Administration (2.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)].
Neonates with a history of in utero exposure to lisinopril
If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
8.5 Geriatric Use
No dosage adjustment with lisinopril is necessary in elderly patients. In a clinical study of lisinopril in patients with myocardial infarctions (GISSI-3 Trial) 4,413 (47%) were 65 and over, while 1,656 (18%) were 75 and over. In this study, 4.8 % of patients aged 75 years and older discontinued lisinopril treatment because of renal dysfunction vs. 1.3% of patients younger than 75 years. No other differences in safety or effectiveness were observed between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Race
ACE inhibitors, including lisinopril, have an effect on blood pressure that is less in black patients than in non blacks.
8.7 Renal Impairment
Dose adjustment of lisinopril is required in patients undergoing hemodialysis or whose creatinine clearance is ≤ 30 mL/min. No dose adjustment of lisinopril is required in patients with creatinine clearance > 30 mL/min [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
10 Overdosage
Following a single oral dose of 20 g/kg no lethality occurred in rats, and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Lisinopril can be removed by hemodialysis [see Clinical Pharmacology (12.3)].
11 Description
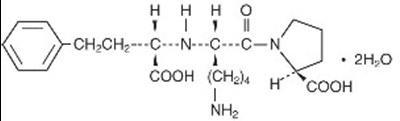
Lisinopril is an oral long-acting angiotensin converting enzyme (ACE) inhibitor. Lisinopril, a synthetic peptide derivative, is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O52H2O and its structural formula is:
Lisinopril, USP is a white to off-white, crystalline powder, with a molecular weight of 441.53. It is soluble in water and sparingly soluble in methanol and practically insoluble in ethanol.
Lisinopril tablets, USP are supplied as 2.5 mg, 5 mg, 10 mg, 20 mg, 30 mg and 40 mg tablets for oral administration.
Inactive Ingredients:
2.5 mg tablets - lactose monohydrate, maize starch, colloidal silicon dioxide, magnesium stearate.
5 mg, 10 mg, 20 mg and 30 mg tablets – lactose monohydrate, maize starch, colloidal silicon dioxide, magnesium stearate, iron oxide red.
40 mg tablets - lactose monohydrate, maize starch, colloidal silicon dioxide, magnesium stearate, iron oxide yellow.
12.1 Mechanism Of Action
Lisinopril inhibits angiotensin-converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. The beneficial effects of lisinopril in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin-aldosterone system. Inhibition of ACE results in decreased plasma angiotensin II which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium. In hypertensive patients with normal renal function treated with lisinopril alone for up to 24 weeks, the mean increase in serum potassium was approximately 0.1 mEq/L; however, approximately 15% of patients had increases greater than 0.5 mEq/L and approximately 6% had a decrease greater than 0.5 mEq/L. In the same study, patients treated with lisinopril and hydrochlorothiazide for up to 24 weeks had a mean decrease in serum potassium of 0.1 mEq/L; approximately 4% of patients had increases greater than 0.5 mEq/L and approximately 12% had a decrease greater than 0.5 mEq/L [see Clinical Studies (14.1)]. Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of lisinopril remains to be elucidated.
While the mechanism through which lisinopril lowers blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, lisinopril is antihypertensive even in patients with low-renin hypertension. Although lisinopril was antihypertensive in all races studied, Black hypertensive patients (usually a low-renin hypertensive population) had a smaller average response to monotherapy than non Black patients.
Concomitant administration of lisinopril and hydrochlorothiazide further reduced blood pressure in Black and non-Black patients and any racial differences in blood pressure response were no longer evident.
12.2 Pharmacodynamics
Hypertension
Adult Patients: Administration of lisinopril to patients with hypertension results in a reduction of both supine and standing blood pressure to about the same extent with no compensatory tachycardia. Symptomatic postural hypotension is usually not observed although it can occur and should be anticipated in volume and/or salt-depleted patients [see Warnings and Precautions (5.4)]. When given together with thiazide-type diuretics, the blood pressure lowering effects of the two drugs are approximately additive.
In most patients studied, onset of antihypertensive activity was seen at one hour after oral administration of an individual dose of lisinopril, with peak reduction of blood pressure achieved by 6 hours. Although an antihypertensive effect was observed 24 hours after dosing with recommended single daily doses, the effect was more consistent and the mean effect was considerably larger in some studies with doses of 20 mg or more than with lower doses; however, at all doses studied, the mean antihypertensive effect was substantially smaller 24 hours after dosing than it was 6 hours after dosing.
The antihypertensive effects of lisinopril are maintained during long-term therapy. Abrupt withdrawal of lisinopril has not been associated with a rapid increase in blood pressure, or a significant increase in blood pressure compared to pretreatment levels.
Non-Steroidal Anti-Inflammatory Agents
In a study in 36 patients with mild to moderate hypertension where the antihypertensive effects of lisinopril alone were compared to lisinopril given concomitantly with indomethacin, the use of indomethacin was associated with a reduced effect, although the difference between the two regimens was not significant.
12.3 Pharmacokinetics
Adult Patients: Following oral administration of lisinopril, peak serum concentrations of lisinopril occur within about 7 hours, although there was a trend to a small delay in time taken to reach peak serum concentrations in acute myocardial infarction patients. Food does not alter the bioavailability of lisinopril. Declining serum concentrations exhibit a prolonged terminal phase, which does not contribute to drug accumulation. This terminal phase probably represents saturable binding to ACE and is not proportional to dose. Upon multiple dosing, lisinopril exhibits an effective half-life of 12 hours.
Lisinopril does not appear to be bound to other serum proteins. Lisinopril does not undergo metabolism and is excreted unchanged entirely in the urine. Based on urinary recovery, the mean extent of absorption of lisinopril is approximately 25%, with large intersubject variability (6% to 60%) at all doses tested (5 mg to 80 mg). The absolute bioavailability of lisinopril is reduced to 16% in patients with stable NYHA Class II to Class IV congestive heart failure, and the volume of distribution appears to be slightly smaller than that in normal subjects. The oral bioavailability of lisinopril in patients with acute myocardial infarction is similar to that in healthy volunteers.
Impaired renal function decreases elimination of lisinopril, which is excreted principally through the kidneys, but this decrease becomes clinically important only when the glomerular filtration rate is below 30 mL/min. Above this glomerular filtration rate, the elimination half-life is little changed. With greater impairment, however, peak and trough lisinopril levels increase, time to peak concentration increases and time to attain steady state is prolonged [see Dosage and Administration (2.4)]. Lisinopril can be removed by hemodialysis.
Pediatric Patients: The pharmacokinetics of lisinopril were studied in 29 pediatric hypertensive patients between 6 years and 16 years with glomerular filtration rate > 30 mL/min/1.73 m2. After doses of 0.1 to 0.2 mg per kg, steady state peak plasma concentrations of lisinopril occurred within 6 hours and the extent of absorption based on urinary recovery was about 28%. These values are similar to those obtained previously in adults. The typical value of lisinopril oral clearance (systemic clearance/absolute bioavailability) in a child weighing 30 kg is 10 L/h, which increases in proportion to renal function. In a multicenter, open-label pharmacokinetic study of daily oral lisinopril in 22 pediatric hypertensive patients with stable kidney transplant (ages 7-17 years; estimated glomerular filtration rate > 30 mL/min/1.73 m2), dose normalized exposures were in the range reported previously in children without a kidney transplant.
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
There was no evidence of a tumorigenic effect when lisinopril was administered for 105 weeks to male and female rats at doses up to 90 mg per kg per day (about 56 or 9 times* the maximum recommended daily human dose, based on body weight and body surface area, respectively). There was no evidence of carcinogenicity when lisinopril was administered for 92 weeks to (male and female) mice at doses up to 135 mg per kg per day (about 84 times* the maximum recommended daily human dose). This dose was 6.8 times the maximum human dose based on body surface area in mice.
Lisinopril was not mutagenic in the Ames microbial mutagen test with or without metabolic activation. It was also negative in a forward mutation assay using Chinese hamster lung cells. Lisinopril did not produce single strand DNA breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, lisinopril did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
There were no adverse effects on reproductive performance in male and female rats treated with up to 300 mg per kg per day of lisinopril. This dose is 188 times and 30 times the maximum human dose when based on mg/kg and mg/m2, respectively.
Studies in rats indicate that lisinopril crosses the blood brain barrier poorly. Multiple doses of lisinopril in rats do not result in accumulation in any tissues. Milk of lactating rats contains radioactivity following administration of 14C lisinopril. By whole body autoradiography, radioactivity was found in the placenta following administration of labeled drug to pregnant rats, but none was found in the fetuses.
*Calculations assume a human weight of 50 kg and human body surface area of 1.62m2
14.1 Hypertension
Two dose-response studies utilizing a once-daily regimen were conducted in 438 mild to moderate hypertensive patients not on a diuretic. Blood pressure was measured 24 hours after dosing. An antihypertensive effect of lisinopril was seen with 5 mg of lisinopril in some patients. However, in both studies blood pressure reduction occurred sooner and was greater in patients treated with 10 mg, 20 mg or 80 mg of lisinopril than patients treated with 5 mg of lisinopril.
In controlled clinical studies of patients with mild to moderate hypertension, patients were treated with lisinopril 20 mg to 80 mg daily, hydrochlorothiazide 12.5 mg to 50 mg daily or atenolol 50 mg to 200 mg daily; and in other studies of patients with moderate to severe hypertension, patients were treated with lisinopril 20 mg to 80 mg daily or metoprolol 100 mg to 200 mg daily. Lisinopril demonstrated superior reductions of systolic and diastolic compared to hydrochlorothiazide in a population that was 75% Caucasian. Lisinopril was approximately equivalent to atenolol and metoprolol in reducing diastolic blood pressure, and had somewhat greater effects on systolic blood pressure.
Lisinopril had similar blood pressure reductions and adverse effects in younger and older (> 65 years) patients. It was less effective in reducing blood pressure in Blacks than in Caucasians.
In hemodynamic studies of lisinopril in patients with essential hypertension, blood pressure reduction was accompanied by a reduction in peripheral arterial resistance with little or no change in cardiac output and in heart rate. In a study in nine hypertensive patients, following administration of lisinopril, there was an increase in mean renal blood flow that was not significant. Data from several small studies are inconsistent with respect to the effect of lisinopril on glomerular filtration rate in hypertensive patients with normal renal function, but suggest that changes, if any, are not large.
In patients with renovascular hypertension lisinopril has been shown to be well tolerated and effective in reducing blood pressure [see Warnings and Precautions (5.3)].
Pediatric Patients: In a clinical study involving 115 hypertensive pediatric patients 6 to 16 years of age, patients who weighed <50 kg received either 0.625 mg, 2.5 mg or 20 mg of lisinopril once daily and patients who weighed >50 kg received either 1.25 mg, 5 mg, or 40 mg of lisinopril once daily. At the end of 2 weeks, lisinopril lowered trough blood pressure in a dose-dependent manner with antihypertensive efficacy demonstrated at doses >1.25 mg (0.02 mg per kg). This effect was confirmed in a randomized withdrawal phase, where the diastolic pressure rose by about 9 mmHg more in patients randomized to placebo than compared to patients who remained on the middle and high doses of lisinopril. The dose-dependent antihypertensive effect of lisinopril was consistent across several demographic subgroups: age, Tanner stage, gender, and race. In this study, lisinopril was generally well-tolerated.
In the above pediatric studies, lisinopril was given either as tablets or in a suspension for those children and infants who were unable to swallow tablets or who required a lower dose than is available in tablet form [see Dosage and Administration (2.1)].
14.2 Heart Failure
In two placebo controlled, 12-week clinical studies compared the addition of lisinopril up to 20 mg daily to digitalis and diuretics alone. The combination of lisinopril, digitalis and diuretics reduced the following signs and symptoms of heart failure: edema, rales, paroxysmal nocturnal dyspnea and jugular venous distention. In one of the studies, the combination of lisinopril, digitalis and diuretics reduced orthopnea, presence of third heart sound and the number of patients classified as NYHA Class III and IV; and improved exercise tolerance. A large (over 3000 patients) survival study, the ATLAS Trial, comparing 2.5 mg and 35 mg of lisinopril in patients with systolic heart failure, showed that the higher dose of lisinopril had outcomes at least as favorable as the lower dose.
During baseline-controlled clinical trials, in patients with systolic heart failure receiving digitalis and diuretics, single doses of lisinopril resulted in decreases in pulmonary capillary wedge pressure, systemic vascular resistance and blood pressure accompanied by an increase in cardiac output and no change in heart rate.
14.3 Acute Myocardial Infarction
The Gruppo Italiano per lo Studio della Sopravvienza nell’Infarto Miocardico (GISSI-3) study was a multicenter, controlled, randomized, unblinded clinical trial conducted in 19,394 patients with acute myocardial infarction (MI) admitted to a coronary care unit. It was designed to examine the effects of short-term (6 week) treatment with lisinopril, nitrates, their combination, or no therapy on short-term (6 week) mortality and on long-term death and markedly impaired cardiac function. Hemodynamically - stable patients presenting within 24 hours of the onset of symptoms were randomized, in a 2 x 2 factorial design, to six weeks of either 1) Lisinopril alone (n=4841), 2) nitrates alone (n=4869), 3) lisinopril plus nitrates (n=4841), or 4) open control (n=4843). All patients received routine therapies, including thrombolytics (72%), aspirin (84%), and a beta blocker (31%), as appropriate, normally utilized in acute myocardial infarction (MI) patients.
The protocol excluded patients with hypotension (systolic blood pressure ≤100 mmHg), severe heart failure, cardiogenic shock, and renal dysfunction (serum creatinine >2 mg per dL and/or proteinuria >500 mg per 24 h). Patients randomized to lisinopril received 5 mg within 24 hours of the onset of symptoms, 5 mg after 24 hours, and then 10 mg daily thereafter. Patients with systolic blood pressure less than 120 mmHg at baseline received 2.5 mg of lisinopril. If hypotension occurred, the lisinopril dose was reduced or if severe hypotension occurred lisinopril was stopped [see Dosage and Administration (2.3)].
The primary outcomes of the trial were the overall mortality at 6 weeks and a combined end point at 6 months after the myocardial infarction, consisting of the number of patients who died, had late (day 4) clinical congestive heart failure, or had extensive left ventricular damage defined as ejection fraction ≤ 35% or an akinetic-dyskinetic [A-D] score > 45%. Patients receiving lisinopril (n=9646), alone or with nitrates, had an 11% lower risk of death (p=0.04) compared to patients who did not receive lisinopril (n=9672) (6.4% vs. 7.2%, respectively) at six weeks. Although patients randomized to receive lisinopril for up to six weeks also fared numerically better on the combined end point at 6 months, the open nature of the assessment of heart failure, substantial loss to follow-up echocardiography, and substantial excess use of lisinopril between 6 weeks and 6 months in the group randomized to 6 weeks of lisinopril, preclude any conclusion about this end point.
Patients with acute myocardial infarction, treated with lisinopril, had a higher (9.0% versus 3.7%) incidence of persistent hypotension (systolic blood pressure < 90 mmHg for more than 1 hour) and renal dysfunction (2.4% versus 1.1%) in-hospital and at six weeks (increasing creatinine concentration to over 3 mg per dL or a doubling or more of the baseline serum creatinine concentration) [see Adverse Reactions (6.1)].
16 How Supplied/Storage And Handling
2.5 mg Tablets: White, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “417” on the other side are supplied in bottle of 30 tablets (NDC 76282-417-30), bottle of 45 tablets (NDC 76282-417-45) bottle of 90 tablets (NDC 76282-417-90) bottle of 100 tablets (NDC 76282-417-01), bottle of 180 tablets (NDC 76282-417-18), bottle of 500 tablets (NDC 76282-417-05) and 1000 tablets (NDC76282-417-10).
5 mg Tablets: Red, round, biconvex, bevel-edged tablets debossed with ‘I’ on the left side of functional score and ‘G’ on the right side of functional score, and “418” on the other side are supplied in bottles of 30 tablets (NDC 76282-418-30), bottle of 45 tablets (NDC 76282-418-45) bottle of 90 tablets (NDC 76282-418-90) bottle of 100 tablets (NDC 76282-418-01), bottle of 180 tablets (NDC 76282-418-18), bottle of 500 tablets (NDC 76282-418-05) and 1000 tablets (NDC 76282-418-10).
10 mg Tablets: Red, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “419” on the other side are supplied in bottles of 30 tablets (NDC 76282-419-30), bottle of 45 tablets (NDC 76282-419-45) bottle of 90 tablets (NDC 76282-419-90) bottle of 100 tablets (NDC 76282-419-01) bottle of 180 tablets (NDC 76282-419- 18), bottle of 500 tablets (NDC 76282-419-05), and 1000 tablets (NDC 76282-419-10).
20 mg Tablets: Red, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “420” on the other side are supplied in bottles of 30 tablets (NDC 76282-420-30), bottle of 45 tablets (NDC 76282-420-45) bottle of 90 tablets (NDC 76282-420-90) bottle of 100 tablets (NDC 76282-420-01), bottle of 180 tablets (NDC 76282-420- 18), bottle of 500 tablets (NDC 76282-420-05) and 1000 tablets (NDC 76282-420-10).
30 mg Tablets: Red, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “421” on the other side are supplied in bottles of 30 tablets (NDC 76282-421-30), bottle of 45 tablets (NDC 76282-421-45) bottle of 90 tablets (NDC 76282-421-90) bottle of 100 tablets (NDC 76282-421-01), bottle of 180 tablets (NDC 76282-421-18), bottle of 500 tablets (NDC 76282-421-05) and 1000 tablets (NDC 76282-421-10).
40 mg Tablets: Yellow, round, biconvex, bevel-edged tablets debossed with ‘IG’ on one side and “422” on the other side are supplied in bottles of 30 tablets (NDC 76282-422-30), bottle of 45 tablets (NDC 76282-422-45) bottle of 90 tablets (NDC 76282-422-90) bottle of 100 tablets (NDC 76282-422-01), bottle of 180 tablets (NDC 76282-422-18), bottle of 500 tablets (NDC 76282-422-05) and 1000 tablets (NDC 76282-422-10).
Store at controlled room temperature, 20° to 25°C (68° to 77°F) [see USP]. Protect from moisture, freezing and excessive heat. Dispense in a tight container.
17 Patient Counseling Information
NOTE: This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Pregnancy: Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to notify their healthcare provider with a known or suspected pregnancy [see Warnings and Precautions (5.1) and Use in specific Populations (8.1)].
Angioedema: Angioedema, including laryngeal edema may occur at any time during treatment with angiotensin converting enzyme inhibitors, including lisinopril. Tell patients to report immediately any signs or symptoms suggesting angioedema (swelling of face, extremities, eyes, lips, tongue, difficulty in swallowing or breathing) and to take no more drug until they have consulted with the prescribing physician.
Lactation: Advise women not to breastfeed during treatment with lisinopril [see Use in Specific Populations (8.2)].
Symptomatic Hypotension: Tell patients to report light-headedness especially during the first few days of therapy. If actual syncope occurs, tell the patient to discontinue the drug until they have consulted with the prescribing physician.
Tell patients that excessive perspiration and dehydration may lead to an excessive fall in blood pressure because of reduction in fluid volume. Other causes of volume depletion such as vomiting or diarrhea may also lead to a fall in blood pressure; advise patients accordingly.
Hyperkalemia: Tell patients not to use salt substitutes containing potassium without consulting their physician.
Hypoglycemia: Tell diabetic patients treated with oral antidiabetic agents or insulin starting an ACE inhibitor to monitor for hypoglycaemia closely, especially during the first month of combined use [see Drug Interactions (7.2)].
Leukopenia/Neutropenia: Tell patients to report promptly any indication of infection (e.g., sore throat, fever), which may be a sign of leukopenia/neutropenia.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Exelan Pharmaceuticals, Inc.
Manufactured for:
Exelan Pharmaceuticals, Inc.
Lawrenceville, GA 30046
Manufactured by:
InvaGen Pharmaceuticals, Inc.
Hauppauge, NY 11788
or
Ascent Pharmaceuticals, Inc.
Central Islip, NY 11722
Revised: 01/2017
Package/Label Display Panel
NDC 76282-417-30
Lisinopril Tablets, USP
2.5 mg
Rx Only 30 Tablets
NDC 76282-418-30
Lisinopril Tablets, USP
5 mg
Rx Only 30 Tablets
NDC 76282-419-30
Lisinopril Tablets, USP
10 mg
Rx Only 30 Tablets
NDC 76282-420-30
Lisinopril Tablets, USP
20 mg
Rx Only 30 Tablets
-
NDC 76282-421-30
Lisinopril Tablets, USP
30 mg
Rx Only 30 Tablets
NDC 76282-422-30
Lisinopril Tablets, USP
40 mg
Rx Only 30 Tablets
* Please review the disclaimer below.