FDA Label for Gallium Ga-68 Psma-11
View Indications, Usage & Precautions
- 1 INDICATIONS AND USAGE
- 2.1 RADIATION SAFETY – DRUG HANDLING
- OTHER
- 2.3 PATIENT PREPARATION PRIOR TO PET IMAGING
- 2.4 IMAGE ACQUISITION
- 2.5 IMAGE INTERPRETATION
- 2.6 RADIATION DOSIMETRY
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5.1 RISK FOR MISDIAGNOSIS
- 5.2 RADIATION RISKS
- CLINICAL TRIALS EXPERIENCE
- 8.4 PEDIATRIC USE
- 8.5 GERIATRIC USE
- 10 OVERDOSAGE
- 11.1 CHEMICAL CHARACTERISTICS
- 11.2 PHYSICAL CHARACTERISTICS
- 11.3 EXTERNAL RADIATION
- 12.1 MECHANISM OF ACTION
- 12.2 PHARMACODYNAMICS
- 13.1 CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- 14 CLINICAL STUDIES
- 16.1 HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL - 12 ML VIAL LABEL
Gallium Ga-68 Psma-11 Product Label
The following document was submitted to the FDA by the labeler of this product Ucla Biomedical Cyclotron. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
1 Indications And Usage
Ga 68 PSMA-11 Injection is indicated for positron emission tomography (PET) of prostate-specific membrane antigen (PSMA) positive lesions in men with prostate cancer:
- with suspected metastasis who are candidates for initial definitive therapy.
- with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level.
2.1 Radiation Safety – Drug Handling
Handle Ga 68 PSMA-11 Injection with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions (5.2)] . Use waterproof gloves, effective radiation shielding, and other appropriate safety measures when preparing and handling Ga 68 PSMA-11 Injection.
Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
Other
Recommended Dosage
In adults, the recommended amount of radioactivity to be administered for PET is 111 MBq to 259 MBq (3 mCi to 7 mCi) administered as an intravenous bolus injection.
Administration
- Use aseptic technique and radiation shielding when withdrawing and administering Ga 68 PSMA-11 Injection.
- Calculate the necessary volume to administer based on calibration time and required dose.
- Inspect Ga 68 PSMA-11 Injection visually for particulate matter and discoloration before administration. Do not use the drug if the solution contains particulate matter or is discolored.
- Ga 68 PSMA-11 Injection may be diluted with sterile 0.9% Sodium Chloride Injection, USP.
- Assay the final dose immediately before administration to the patient in a dose calibrator.
- After injection of Ga 68 PSMA-11 Injection, administer an intravenous flush of sterile 0.9% Sodium Chloride Injection, USP to ensure full delivery of the dose.
- Dispose of any unused drug in a safe manner in compliance with applicable regulations.
- Unless contraindicated, a diuretic expected to act within the uptake time period may be administered at the time of radiotracer injection to potentially decrease artifact from radiotracer accumulation in the urinary bladder and ureters.
Androgen deprivation therapy and other therapies targeting the androgen pathway
Androgen deprivation therapy (ADT) and other therapies targeting the androgen pathway, such as androgen receptor antagonists, can result in changes in uptake of Ga 68 PSMA-11 in prostate cancer. The effect of these therapies on performance of Ga 68 PSMA-11 PET has not been established.
Risk Summary
Ga 68 PSMA-11 Injection is not indicated for use in females. There are no available data with Ga 68 PSMA-11 Injection use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. All radiopharmaceuticals, including Ga 68 PSMA-11 Injection, have the potential to cause fetal harm depending on the fetal stage of development and the magnitude of the radiation dose. Animal reproduction studies have not been conducted with Ga 68 PSMA-11 Injection.
Risk Summary
Ga 68 PSMA-11 Injection is not indicated for use in females. There are no data on the presence of Ga 68 PSMA-11 in human milk, the effect on the breastfed infant, or the effect on milk production.
Distribution
Intravenously injected Ga 68 PSMA-11 is cleared from the blood and is accumulated preferentially in the liver (15%), kidneys (7%), spleen (2%), and salivary glands (0.5%). Ga 68 PSMA-11 uptake is also seen in the adrenals and prostate. There is no uptake in the cerebral cortex or in the heart, and usually lung uptake is low.
Elimination
A total of 14% of the injected dose is excreted in urine in the first 2 hours post-injection.
PSMA-PreRP
This two-center study enrolled 325 patients with biopsy-proven prostate cancer who were considered candidates for prostatectomy and pelvic lymph node dissection. All enrolled patients met at least one of the following criteria: serum prostate-specific antigen (PSA) of at least 10 ng/mL, tumor stage cT2b or greater, or Gleason score greater than 6. Each patient received a single Ga 68 PSMA-11 PET/CT or PET/MR from mid-thigh to skull base.
A total of 123 patients (38%) proceeded to standard-of-care prostatectomy and template pelvic lymph node dissection and had sufficient histopathology data for evaluation (evaluable patients). Three members of a pool of six central readers independently interpreted each PET scan for the presence of abnormal Ga 68 PSMA-11 uptake in pelvic lymph nodes located in the common iliac, external iliac, internal iliac, and obturator subregions bilaterally as well as in any other pelvic location. The readers were blinded to all clinical information except for the history of prostate cancer prior to definitive treatment. Extrapelvic sites and the prostate gland itself were not analyzed in this study. For each patient, Ga 68 PSMA-11 PET results and reference standard histopathology obtained from dissected pelvic lymph nodes were compared by region (left hemipelvis, right hemipelvis, and other).
For the 123 evaluable patients, the mean age was 65 years (range 45 to 76 years), and 89% were white. The median serum PSA was 11.8 ng/mL. The summed Gleason score was 7 for 44%, 8 for 20%, and 9 for 31% of the patients, with the remainder of the patients having Gleason scores of 6 or 10.
Table 5 compares majority PET reads to pelvic lymph node histopathology results at the patient-level with region matching, such that at least one true positive region defines a true positive patient. As shown, approximately 24% of subjects studied were found to have pelvic nodal metastases based on histopathology (95% confidence interval: 17%, 32%).
| Histopathology | Predictive value
PPV: positive predictive value, NPV: negative predictive value (95% CI) | |||
|---|---|---|---|---|
| Positive | Negative | |||
| PET scan | Positive | 14 | 9 | PPV
61% (41%, 81%) |
| Negative | 16 | 84 | NPV
84% (79%, 91%) | |
| Total | 30 | 93 | ||
| Diagnostic performance
(95% CI) | Sensitivity
47% (29%, 65%) | Specificity
90% (84%, 96%) | ||
Among the pool of six readers, sensitivity ranged from 36% to 60%, specificity from 83% to 96%, positive predictive value from 38% to 80%, and negative predictive value from 80% to 88%.
In an exploratory subgroup analysis based on summed Gleason score, there was a numerical trend toward more true positives in patients with Gleason score of 8 or higher compared to those with Gleason score of 7 or lower.
An exploratory analysis was performed to estimate the sensitivity and specificity for pelvic nodal metastasis detection in all scanned patients, including the patients who were lacking histopathology reference standard. An imputation method was used based on patient-specific factors. This exploratory analysis resulted in an imputed sensitivity of 47%, with a 95% confidence interval ranging from 38% to 55%, and an imputed specificity of 74%, with a 95% confidence interval ranging from 68% to 80% for all patients imaged with Ga 68 PSMA-11 PET.
PSMA-BCR
This two-center study enrolled 635 patients with biochemical evidence of recurrent prostate cancer after definitive therapy, defined by serum PSA of >0.2 ng/mL more than 6 weeks after prostatectomy or by an increase in serum PSA of at least 2 ng/mL above nadir after definitive radiotherapy. All patients received a single Ga 68 PSMA-11 PET/CT or PET/MR from mid-thigh to skull base. Three members of a pool of nine independent central readers evaluated each scan for the presence and regional location (20 subregions grouped into four regions) of abnormal Ga 68 PSMA-11 uptake suggestive of recurrent prostate cancer. The readers were blinded to all clinical information other than type of primary therapy and most recent serum PSA level.
A total of 469 patients (74%) had at least one positive region detected by Ga 68 PSMA-11 PET majority read. The distribution of Ga 68 PSMA-11 PET positive regions was 34% bone, 25% prostate bed, 25% pelvic lymph node, and 17% extrapelvic soft tissue. Two hundred and ten patients had composite reference standard information collected in a PET positive region (evaluable patients), consisting of at least one of the following: histopathology, imaging (bone scintigraphy, CT, or MRI) acquired at baseline or within 12 months after Ga 68 PSMA-11 PET, or serial serum PSA. Composite reference standard information for Ga 68 PSMA-11 PET negative regions was not systematically collected in this study.
In the 210 evaluable patients, the mean age was 70 years (range 49 to 88 years) and 82% were 65 years of age or older. White patients made up 90% of the group. The median serum PSA was 3.6 ng/mL. Prior treatment included radical prostatectomy in 64% and radiotherapy in 73%.
Of the 210 evaluable patients, 192 patients (91%) were found to be true positive in one or more regions against the composite reference standard (95% confidence interval: 88%, 95%). Among the pool of nine readers used in the study, the proportion of patients who were true positive in one or more regions ranged from 82% to 97%. The prostate bed had the lowest proportion of true positive results at the region-level (76% versus 96% for non-prostate regions).
An exploratory analysis was also performed in which Ga 68 PSMA-11 PET positive patients who lacked reference standard information were imputed using an estimated likelihood that at least one location-matched PET positive lesion was reference standard positive based on patient-specific factors. In this exploratory analysis, 340 of 475 patients (72%) were imputed as true positive in one or more regions (95% confidence interval: 68%, 76%).
In another exploratory analysis using the same imputation approach for PET positive patients who lacked reference standard information, 340 of 635 patients (54%) were correctly detected as true positive (95% confidence interval: 50%, 57%) among all BCR patients who received a PET scan, whether it was read as positive or negative.
The likelihood of identifying a Ga 68 PSMA-11 PET positive lesion in this study generally increased with higher serum PSA level. Table 6 shows the patient-level Ga 68 PSMA-11 PET results stratified by serum PSA level. The mean time between PSA measurement and PET scan was 40 days with a range of 0 to 367 days. Percent PET positivity was calculated as the proportion of patients with a positive Ga 68 PSMA-11 PET out of all patients scanned. Percent PET positivity includes patients determined to be either true positive or false positive as well as those in whom such determination was not made due to the absence of composite reference standard data.
| PSA
(ng/mL) | PET positive patients | PET negative patients | Percent PET positivity
Percent PET positivity = PET positive patients/total patients scanned (95% CI) | |||
|---|---|---|---|---|---|---|
| Total | TP
TP: true positive, FP: false positive | FP
| Without reference standard | |||
| With reference standard | ||||||
| <0.5 | 48 | 11 | 1 | 36 | 87 | 36%
(27%, 44%) |
| 12 | ||||||
| ≥0.5 and <1 | 44 | 15 | 3 | 26 | 35 | 56%
(45%, 67%) |
| 18 | ||||||
| ≥1 and <2 | 71 | 29 | 1 | 41 | 15 | 83%
(75%, 91%) |
| 30 | ||||||
| ≥2 | 299 | 137 | 13 | 149 | 29 | 91%
(88%, 94%) |
| 150 | ||||||
| Total | 462 | 192 | 18 | 252 | 166 | 74%
(70%, 77%) |
| 210 | ||||||
Storage
Store Ga 68 PSMA-11 Injection upright in a lead shielded container at 25°C (77°F); excursions are permitted from 15°C to 30°C (59°F to 86°F). Store Ga 68 PSMA-11 Injection within the original container in radiation shielding.
Handling
Receipt, transfer, handling, possession, or use of this product is subject to the radioactive material regulations and licensing requirements of the U.S. Nuclear Regulatory Commission, Agreement States, or Licensing States as appropriate.
Adequate Hydration
Instruct patients to drink a sufficient amount of water to ensure adequate hydration before their PET study and urge them to drink and urinate as often as possible during the first hours following the administration of Ga 68 PSMA-11 Injection, in order to reduce radiation exposure [see Dosage and Administration (2.3) and Warnings and Precautions (5.2)] .
Manufactured and Distributed by:
University of California, Los Angeles
UCLA Biomedical Cyclotron Facility
780 Westwood Plaza
Los Angeles, CA 90095
(310) 794-7638
2.3 Patient Preparation Prior To Pet Imaging
Instruct patients to drink a sufficient amount of water to ensure adequate hydration prior to administration of Ga 68 PSMA-11 Injection and to continue to drink and void frequently following administration to reduce radiation exposure, particularly during the first hour after administration [see Warnings and Precautions (5.2)] .
2.4 Image Acquisition
Position the patient supine with arms above the head. Begin PET scanning 50 to 100 minutes after the intravenous administration of Ga 68 PSMA-11 Injection. Patients should void immediately prior to image acquisition and that image acquisition should begin at the proximal thighs and proceed cranially to the skull base or skull vertex. Adapt imaging technique according to the equipment used and patient characteristics in order to obtain the best image quality possible.
2.5 Image Interpretation
Ga 68 PSMA-11 binds to prostate-specific membrane antigen (PSMA). Based on the intensity of the signals, PET images obtained using Ga 68 PSMA-11 Injection indicate the presence of PSMA in tissues. Lesions should be considered suspicious if uptake is greater than physiologic uptake in that tissue or greater than adjacent background if no physiologic uptake is expected. Tumors that do not bear PSMA will not be visualized. Increased uptake in tumors is not specific for prostate cancer [see Warnings and Precautions (5.1)] .
2.6 Radiation Dosimetry
Estimated radiation absorbed doses per injected activity for organs and tissues of adult male patients following an intravenous bolus of Ga 68 PSMA-11 Injection are shown in Table 1.
The effective radiation dose resulting from the administration of 259 MBq (7 mCi) is about 4.4 mSv. The radiation doses for this administered dose to the critical organs, which are the kidneys, urinary bladder, and spleen, are 96.2 mGy, 25.4 mGy, and 16.8 mGy, respectively.
These radiation doses are for Ga 68 PSMA-11 Injection alone. If CT or a transmission source are used for attenuation correction, the radiation dose will increase by an amount that varies by technique.
| Organ | Absorbed dose (mGy/MBq) | |
|---|---|---|
| Mean | SD | |
| Adrenals | 0.0156 | 0.0014 |
| Brain | 0.0104 | 0.0011 |
| Breasts | 0.0103 | 0.0011 |
| Gallbladder | 0.0157 | 0.0012 |
| Lower Colon | 0.0134 | 0.0009 |
| Small Intestine | 0.0140 | 0.0020 |
| Stomach | 0.0129 | 0.0008 |
| Heart | 0.0120 | 0.0009 |
| Kidneys | 0.3714 | 0.0922 |
| Liver | 0.0409 | 0.0076 |
| Lungs | 0.0111 | 0.0007 |
| Muscle | 0.0103 | 0.0003 |
| Pancreas | 0.0147 | 0.0009 |
| Red Marrow | 0.0114 | 0.0016 |
| Skin | 0.0091 | 0.0003 |
| Spleen | 0.0650 | 0.0180 |
| Testes | 0.0111 | 0.0006 |
| Thymus | 0.0105 | 0.0006 |
| Thyroid | 0.0104 | 0.0006 |
| Urinary Bladder | 0.0982 | 0.0286 |
| Total Body | 0.0143 | 0.0013 |
| Effective Dose (mSv/MBq) | 0.0169 | 0.0015 |
3 Dosage Forms And Strengths
Injection: supplied as a clear, colorless solution in a 30 mL multiple-dose vial containing 18.5 MBq/mL to 185 MBq/mL (0.5 mCi/mL to 5 mCi/mL) of Ga 68 PSMA-11 at calibration time.
4 Contraindications
None
5.1 Risk For Misdiagnosis
Image interpretation errors can occur with Ga 68 PSMA-11 PET. A negative image does not rule out the presence of prostate cancer and a positive image does not confirm the presence of prostate cancer. The performance of Ga 68 PSMA-11 Injection for imaging of biochemically recurrent prostate cancer seems to be affected by serum PSA levels and by site of disease [See Clinical Studies (14)] . The performance of Ga 68 PSMA-11 Injection for imaging of metastatic pelvic lymph nodes prior to initial definitive therapy seems to be affected by Gleason score [See Clinical Studies (14)] . Ga 68 PSMA-11 uptake is not specific for prostate cancer and may occur with other types of cancer as well as non-malignant processes such as Paget's disease, fibrous dysplasia, and osteophytosis. Clinical correlation, which may include histopathological evaluation of the suspected prostate cancer site, is recommended.
5.2 Radiation Risks
Ga 68 PSMA-11 Injection contributes to a patient's overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased risk for cancer. Ensure safe handling to minimize radiation exposure to the patient and health care workers. Advise patients to hydrate before and after administration and to void frequently after administration [see Dosage and Administration (2.1, 2.3)] .
Clinical Trials Experience
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of Ga 68 PSMA-11 Injection was evaluated in 960 patients, each receiving one dose of Ga 68 PSMA-11 Injection. The average injected activity was 188.7 ± 40.7 MBq (5.1 ± 1.1 mCi).
No serious adverse reactions were attributed to Ga 68 PSMA-11 Injection. The most commonly reported adverse reactions were nausea, diarrhea, and dizziness, occurring at a rate of < 1%.
8.4 Pediatric Use
Ga 68 PSMA-11 Injection is not indicated for use in the pediatric population. There are no studies of Ga 68 PSMA-11 Injection in pediatric patients.
8.5 Geriatric Use
The efficacy of Ga 68 PSMA-11 PET in geriatric patients with prostate cancer is based on data from two prospective studies [see Clinical Studies (14)] . Most patients in these trials were 65 years of age or older (72%). The efficacy and safety profiles of Ga 68 PSMA-11 Injection appear similar in adult and geriatric patients with prostate cancer, although the number of adult patients in the trials was not large enough to allow definitive comparison.
10 Overdosage
In the event of an overdose of Ga 68 PSMA-11 Injection, reduce the radiation absorbed dose to the patient where possible by increasing the elimination of the drug from the body using hydration and frequent bladder voiding. A diuretic might also be considered. If possible, an estimate of the radiation effective dose given to the patient should be made.
11.1 Chemical Characteristics
Ga 68 PSMA-11 Injection is a radioactive diagnostic agent for intravenous administration. It contains 5 mcg PSMA-11, 18.5 MBq/mL to 185 MBq/mL (0.5 mCi/mL to 5 mCi/mL) of Ga 68 PSMA-11 at calibration time, 1 mL ethanol, 1 mL water for injection, and 10 mL of 0.9% sodium chloride solution (approximately 12 mL total volume). Ga 68 PSMA-11 Injection is provided as a sterile, pyrogen free, clear, colorless solution for intravenous use, with a pH between 4.0 and 7.0.
Ga 68 PSMA-11 is a urea based peptidomimetic that has a covalently bound chelator (HBED-CC). The peptide has the amino acid sequence Glu-NH-CO-NH-Lys(Ahx)-HBED-CC. Ga 68 PSMA-11 has a molecular weight of 1011.91 g/mol and its chemical structure is shown in Figure 1.
Figure 1: Chemical Structure of Ga 68 PSMA-11
11.2 Physical Characteristics
Gallium-68 (Ga 68) decays with a half-life of 68 minutes to stable zinc-68. Table 2 and Table 3 display the principle radiation emission data and physical decay of Ga 68.
| Radiation/ Emission | % Disintegration | Mean Energy
(MeV) |
|---|---|---|
| beta+ | 88% | 0.8360 |
| beta+ | 1.1% | 0.3526 |
| gamma | 178% | 0.5110 |
| gamma | 3.0% | 1.0770 |
| X-ray | 2.8% | 0.0086 |
| X-ray | 1.4% | 0.0086 |
| Minutes | Fraction Remaining |
|---|---|
| 0 | 1 |
| 15 | 0.858 |
| 30 | 0.736 |
| 60 | 0.541 |
| 90 | 0.398 |
| 120 | 0.293 |
| 180 | 0.158 |
| 360 | 0.025 |
11.3 External Radiation
Table 4 displays the radiation attenuation by lead shielding of Ga 68.
| Shield Thickness (Pb) mm | Coefficient of Attenuation |
|---|---|
| 6 | 0.5 |
| 12 | 0.25 |
| 17 | 0.1 |
| 34 | 0.01 |
| 51 | 0.001 |
12.1 Mechanism Of Action
Ga 68 PSMA-11 binds to prostate-specific membrane antigen (PSMA). It binds to cells that express PSMA, including malignant prostate cancer cells, which usually overexpress PSMA. Gallium-68 (Ga 68) is a β+ emitting radionuclide that allows positron emission tomography (PET).
12.2 Pharmacodynamics
The relationship between Ga 68 PSMA-11 plasma concentrations and successful imaging was not explored in clinical trials.
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
No long-term animal studies were performed to evaluate the carcinogenicity potential of Ga 68 PSMA-11 Injection.
14 Clinical Studies
The safety and efficacy of Ga 68 PSMA-11 Injection were established in two prospective, open-label studies (PSMA-PreRP and PSMA-BCR) in men with prostate cancer.
16.1 How Supplied
Ga 68 PSMA-11 Injection (NDC 76394-2642-3) is a clear, colorless solution, supplied in a capped glass vial containing 18.5 MBq/mL to 185 MBq/mL (0.5 mCi/mL to 5 mCi/mL) of Ga 68 PSMA-11 at end of synthesis, in approximately 12 mL. The contents of each vial are sterile, pyrogen-free and preservative-free. The expiration date and time are provided on the container label. Use Ga 68 PSMA-11 Injection within 3 hours of end of synthesis time.
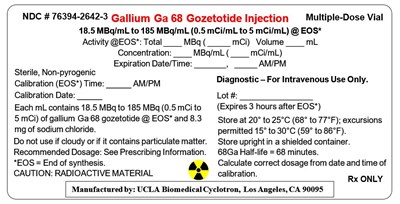
Principal Display Panel - 12 Ml Vial Label
NDC # 76394-2642-3
Ga 68 PSMA-11 Injection
Multiple-Dose Vial
18.5 MBq/mL to 185 MBq/mL (0.5 mCi/mL to 5 mCi/mL) @ EOS*
Activity @EOS*: Total ____ MBq ( _____ mCi) Volume ____ mL
Concentration: ____ MBq/mL ( ____ mCi/mL)
Expiration Date/Time: _______, _____ AM/PM
Sterile, Non-pyrogenic
Calibration (EOS*) Time: _____ AM/PM
Calibration Date: _____
Each mL contains 18.5 MBq to 185 MBq (0.5 mCi to
5 mCi) of Ga 68 PSMA-11 @ EOS* and 8.3 mg of
sodium chloride.
Do not use if cloudy or if it contains particulate matter.
Recommended Dosage: See Prescribing Information.
*EOS = End of synthesis.
CAUTION: RADIOACTIVE MATERIAL
Diagnostic – For Intravenous Use Only.
Lot #: ________________
(Expires 3 hours after EOS*)
Store at 20°-25°C (68°-77°F);
excursions permitted to 15-30°C (59-86°F).
Store upright in a shielded container.
68Ga Half-life = 68 minutes.
Calculate correct dosage from date and time of
calibration.
Rx ONLY
Manufactured by: UCLA Biomedical Cyclotron, Los Angeles, CA 90095
* Please review the disclaimer below.