FDA Label for Maple Cross Hand Sanitizer
View Indications, Usage & Precautions
- DRUG FACTS:
- MEDICINAL / ACTIVE INGREDIENT:
- PURPOSE:
- NON MEDICINAL / INACTIVE INGREDIENTS:
- INDICATIONS:
- DIRECTIONS:
- WARNING:
- KEEP OUT OF REACH OF CHILDREN
- STOP USE AND ASK/ CONSULT A DOCTOR/ PHYSICIAN/ HEALTH CARE PRACTITIONER/ HEALTH CARE ROVIDER/ HEALTH CARE PROFESSIONAL
- WHEN USING THIS PRODUCT
- CONTRAINDICATIONS:
- OTHER INFORMATION:
- PACKAGE LABELING: 300ML
- PACKAGE LABELING: 500ML
- PACKAGE LABELING: 1000ML
Maple Cross Hand Sanitizer Product Label
The following document was submitted to the FDA by the labeler of this product Maple Cross Health Inc.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
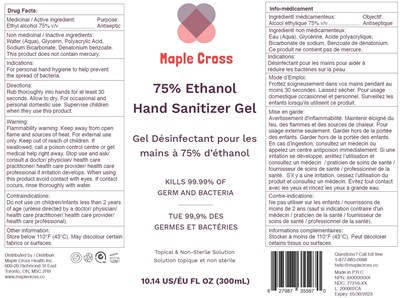
Drug Facts:
Medicinal / Active Ingredient:
Ethyl alcohol 75% v/v
Purpose:
Antiseptic
Non Medicinal / Inactive Ingredients:
Water (Aqua), Glycerin, Polyacrylic Acid,Sodium Bicarbonate, Denatonium benzoate. This product does not contain mercury.
Indications:
For personal hand hygiene to help prevent the spread of bacteria.
Directions:
Rub thoroughly into hands for at least 30 seconds. Allow to dry. For occasional and personal domestic use. Supervise children when they use this product.
Warning:
Flammability warning. Keep away from open flame and sources of heat. For external use only.
Keep Out Of Reach Of Children
If swallowed, call a poison control centre or get medical help right away.
Stop Use And Ask/ Consult A Doctor/ Physician/ Health Care Practitioner/ Health Care Rovider/ Health Care Professional
if irritation develops.
When Using This Product
avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Contraindications:
Do not use on children/infants less than 2 years of age (unless directed by a doctor/ physician/ health care practitioner/ health care provider/
health care professional).
Other Information:
Store below 110°F (43°C). May discolour certain fabrics or surfaces.
Package Labeling: 300Ml
Package Labeling: 500Ml
Package Labeling: 1000Ml
* Please review the disclaimer below.