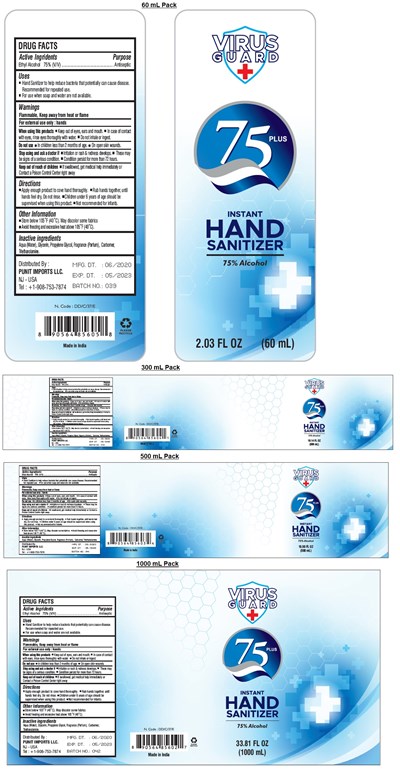
FDA Label for Virus Guard 75 Plus Instant Hand Sanitizer
View Indications, Usage & Precautions
Virus Guard 75 Plus Instant Hand Sanitizer Product Label
The following document was submitted to the FDA by the labeler of this product Milan Cosmetics Private Limited. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Drug Facts
Active Ingredients
Ethyl Alcohol 75% (v/v)
Purpose
Antiseptic
Uses
▪ Hand Sanitizer to help reduce bacteria that potentially can cause disease. Recommended for repeated use.
▪ For use when soap and water are not available.
Warnings
Flammable. Keep away from fire or flame
For external use only: hands
When using this product ▪ Keep out of eyes, ears and mouth. ▪ In case of contact with eyes, rinse eyes thoroughly with water. ▪ Do not inhale or ingest.
Do not use ▪ In children less than 2 months of age. ▪ On open skin wounds.
Stop use and ask a doctor if ▪ Irritation or rash & redness develops. ▪ These may be signs of a serious condition. ▪ Condition persist for more than 72 hours.
Directions
▪ Apply enough product to cove hand thoroughly. ▪ Rub hands together, until hands feel dry, Do not rinse. ▪ Children under 6 years of age should be supervised when using this product. ▪ Not recommended for infants.
Other Information
▪ Store below 105°F (40°C). May discolor some fabrics
▪ Avoid freezing and excessive heat above 105°F (40°C).
Inactive Ingredients
Aqua (Water), Glycerin, Propylene Glycol, Fragrance (Parfum), Carbomer, Triethanolamine.
Packaging
* Please review the disclaimer below.