Product Images Levalbuterol
View Photos of Packaging, Labels & Appearance
- Figure 1 - levalbuterol fig1
- Figure F.1, F.2 - levalbuterol fig10
- levalbuterol-pouch-0.31mg - levalbuterol fig11
- levalbuterol-carton-0.31 - levalbuterol fig12
- levalbuterol-pouch-0.63mg - levalbuterol fig13
- levalbuterol-carton-0.63 - levalbuterol fig14
- levalbuterol-pouch-1.25mg - levalbuterol fig15
- levalbuterol-carton-1.25mg - levalbuterol fig16
- Figure 2 - levalbuterol fig2
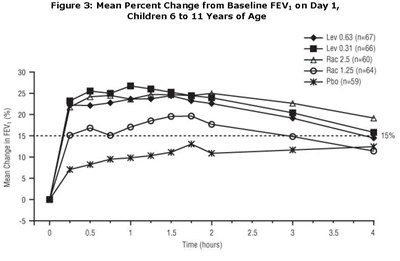
- Figure 3 - levalbuterol fig3
- Figure 4 - levalbuterol fig4
- Figure A - levalbuterol fig5
- Figure B - levalbuterol fig6
- Figure C - levalbuterol fig7
- Figure D.1, D.2 - levalbuterol fig8
- Figure E - levalbuterol fig9
- Levalbuterol Hydrochloride Chemical Structure - levalbuterol str
Product Label Images
The following 17 images provide visual information about the product associated with Levalbuterol NDC 81894-101 by Luoxin Aurovitas Pharma (chengdu) Co., Ltd., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
levalbuterol-carton-0.31 - levalbuterol fig12

This is a description of Levalbuterol Inhalation Solution, USP in 0.31 mg/3 mL strength, packaged in sterile unit-dose vials. Each vial contains 0.31 mg of levalbuterol (equivalent to levalbuterol hydrochloride USP 0.36 mg) and is designed for oral inhalation only. The solution does not contain preservatives and should be used as directed by a physician, with dosage not to be exceeded. The product should be protected from light and stored at 20° to 25°C. Unit-dose vials need to be stored in the provided foil pouch, and once opened, vials are recommended to be used within two weeks. The manufacturer is Aurobindo Pharma USA, Inc., distributed in the US.*
levalbuterol-carton-0.63 - levalbuterol fig14

This is a description of a pharmaceutical product - Levalbuterol Inhalation Solution in 3 mL unit-dose vials. Each vial contains 0.63 mg of levalbuterol. The solution is for oral inhalation only and does not contain preservatives. It is recommended to store the vials in the protective foil pouch and discard if the solution is not colorless. The product should be used within two weeks after opening the foil pouch and within one week after removing the vial from the pouch. This medication is distributed by Aurobindo Pharma USA, Inc. and manufactured in Chengdu, China. It should be used as directed by a physician and kept out of reach of children.*
levalbuterol-pouch-1.25mg - levalbuterol fig15

This text provides detailed information about a medication called Levalbuterol Inhalation Solution, USP, which comes in unit-dose vials containing 1.25 mg of levalbuterol. It includes instructions for storage, handling, and usage, as well as precautions for pharmacists and patients. The medication does not contain preservatives and is meant for oral inhalation only. It is manufactured by Aurobindo Pharma USA, Inc. and Luoxin Aurovitas Pharma in China.*
levalbuterol-carton-1.25mg - levalbuterol fig16

This text describes a medication called Levalbuterol Inhalation Solution, USP, which is available in unit-dose vials containing 1.25 mg of levalbuterol. The solution is for oral inhalation only and each vial should be used within the specified timeframe after opening. The solution contains no preservatives and should be stored as per the given instructions. The manufacturer is Aurobindo Pharma and the product is distributed in the USA. The text includes details on potency, storage, manufacturer's information, and guidelines for pharmacists and patients.*
Figure 2 - levalbuterol fig2
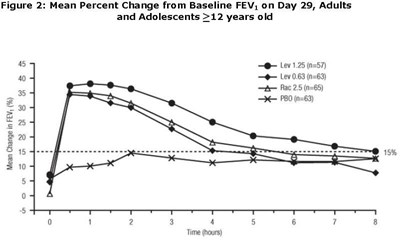
This text provides data on the mean percent change from baseline Forced Expiratory Volume (FEV) on Day 29 for adults and adolescents over 12 years old. It includes information for different treatments (Lev1.25, Lev 063, Rac 25, and PBO) with sample sizes for each group. Additionally, the text shows a 15% mean change in FEV over time.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.