Product Images Ambrisentan
View Photos of Packaging, Labels & Appearance
- image - 47d4a758 e84f 479d affa b6a666c70173 01
- image - 47d4a758 e84f 479d affa b6a666c70173 02
- Image - 47d4a758 e84f 479d affa b6a666c70173 03
- Image - 47d4a758 e84f 479d affa b6a666c70173 04
- image - 47d4a758 e84f 479d affa b6a666c70173 05
- image - 47d4a758 e84f 479d affa b6a666c70173 06
- 5 mg - 47d4a758 e84f 479d affa b6a666c70173 07
- 10 mg - 47d4a758 e84f 479d affa b6a666c70173 08
Product Label Images
The following 8 images provide visual information about the product associated with Ambrisentan NDC 82009-142 by Quallent Pharmaceuticals Health Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
image - 47d4a758 e84f 479d affa b6a666c70173 02
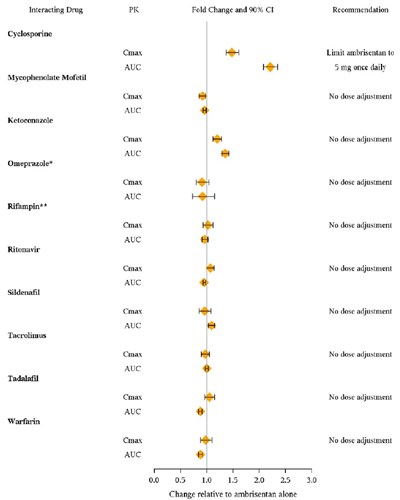
This is a list of interacting drugs including Cyclosporine, Mycophenolate Mofetil, Ketoconazale, Omeprazole, Rifampin, Ritonavir, Sildenafil, Tacrolimus, Tadalafil, and Warfarin. The text provides recommendations for dose adjustments when combined with Ambrisentan. It suggests limiting Ambrisentan to 5 mg once daily and indicates no dose adjustments needed for the other drugs listed.*
Image - 47d4a758 e84f 479d affa b6a666c70173 03
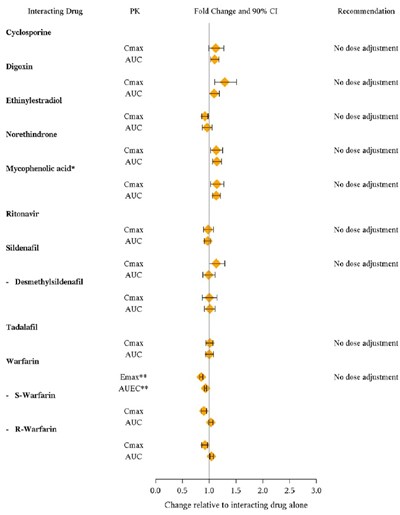
This is a list of interacting drugs and their impact on various pharmacokinetic parameters like Cmax and AUC. It also includes recommendations for dose adjustments based on the interactions observed. The list covers drugs such as Cyclosporine, Digoxin, Ethinylestradiol, Norethindrone, Mycophenolic acid, Ritonavir, Sildenafil, Tadalafil, Warfarin, and provides recommendations for each based on the observed interactions.*
Image - 47d4a758 e84f 479d affa b6a666c70173 04

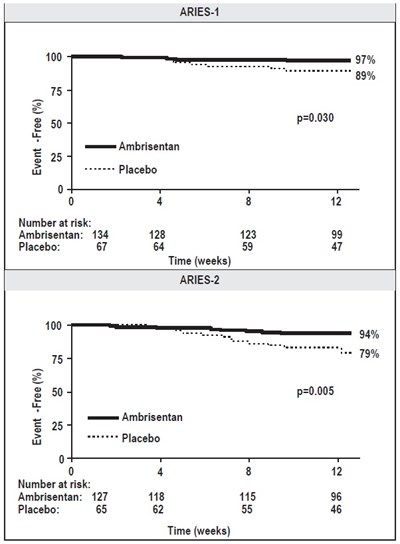
This is a comparison between two groups, ARIES-1 and ARIES-2, over a 12-week period based on meters covered at Weeks 4, 8, and 12. The text indicates that there is a placebo group included in ARIES-2.*
5 mg - 47d4a758 e84f 479d affa b6a666c70173 07

This text provides information about a package of 30 tablets, each containing 5 mg of Ambrisentan. The instructions indicate that the tablets are film-coated and should not be split, crushed, or chewed. It advises storing the tablets at a temperature between 20°C to 25°C (68°F to 77°F). The package is child-resistant and should be kept tightly closed. Additionally, it mentions the availability of a Medication Guide at quallentpharmaceuticals.com or by calling 1-877-605-7243. The tablets are manufactured by Zydus Lifesciences Ltd in Ahmedabad, India, for Quallent Pharmaceuticals in Grand Cayman, Cayman Islands.*
10 mg - 47d4a758 e84f 479d affa b6a666c70173 08
These tablets contain Ambrisentan, 10 mg, and come in a package that is child-resistant. It is important not to spill, crush, or chew the tablets. The recommended storage temperature is between 20°C to 25°C (68 to 77°F). The medication guide is available at quallentpharmaceuticals.com, or you may call 1-677-605-7243 for more information about this prescription drug. The tablets are manufactured by Zydus Lifesciences Ltd in Ahmedabad, India, and distributed by Quallent Pharmaceuticals Health LLC in the Grand Cayman, Cayman Islands.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.