FDA Label for Gomekli
View Indications, Usage & Precautions
- 1 INDICATIONS AND USAGE
- 2.1 RECOMMENDED EVALUATION AND TESTING BEFORE INITIATING GOMEKLI
- 2.2 GOMEKLI DOSAGE FORM OVERVIEW
- 2.3 RECOMMENDED DOSAGE
- 2.4 GOMEKLI PREPARATION AND ADMINISTRATION INSTRUCTIONS
- 2.5 DOSAGE MODIFICATIONS FOR ADVERSE REACTIONS
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5.1 OCULAR TOXICITY
- 5.2 LEFT VENTRICULAR DYSFUNCTION
- 5.3 DERMATOLOGIC ADVERSE REACTIONS
- 5.4 EMBRYO-FETAL TOXICITY
- 6 ADVERSE REACTIONS
- 6.1 CLINICAL TRIALS EXPERIENCE
- 8.1 PREGNANCY
- 8.2 LACTATION
- 8.3 FEMALES AND MALES OF REPRODUCTIVE POTENTIAL
- 8.4 PEDIATRIC USE
- 8.5 GERIATRIC USE
- 8.6 RENAL IMPAIRMENT
- 8.7 HEPATIC IMPAIRMENT
- 11 DESCRIPTION
- 12.1 MECHANISM OF ACTION
- 12.2 PHARMACODYNAMICS
- 12.3 PHARMACOKINETICS
- 13.1 CARCINOGENESIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
- 14.1 NEUROFIBROMATOSIS TYPE 1 ASSOCIATED PLEXIFORM NEUROFIBROMAS
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PRINCIPAL DISPLAY PANEL
Gomekli Product Label
The following document was submitted to the FDA by the labeler of this product Springworks Therapeutics, Inc.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
1 Indications And Usage
GOMEKLI is indicated for the treatment of adult and pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) not amenable to complete resection [see Clinical Studies (14)].
2.1 Recommended Evaluation And Testing Before Initiating Gomekli
Prior to administration of GOMEKLI:
- conduct comprehensive ophthalmic assessment [see Warnings and Precautions (5.1)].
- assess ejection fraction (EF) by echocardiogram [see Warnings and Precautions (5.2)].
2.2 Gomekli Dosage Form Overview
GOMEKLI is available in 2 dosage forms: capsules or tablets for oral suspension.
- GOMEKLI capsules: must be swallowed whole, do not open, break or chew capsules.
- GOMEKLI tablets for oral suspension: can be swallowed whole or can be dispersed in drinking water and administered orally as a liquid [see Dosage and Administration (2.4)].
2.3 Recommended Dosage
The recommended dosage of GOMEKLI is 2 mg/m2 orally twice daily (approximately every 12 hours) with or without food for the first 21 days of each 28-day cycle. The maximum dose is 4 mg twice daily. Continue treatment with GOMEKLI until disease progression or unacceptable toxicity. The recommended dose of GOMEKLI is based on body surface area (BSA) as shown in Table 1.
| Body Surface Area (m2) * | Recommended Dosage for Capsules or Tablets for Oral Suspension |
| 0.40 to 0.69 | 1 mg twice daily |
| 0.70 to 1.04 | 2 mg twice daily |
| 1.05 to 1.49 | 3 mg twice daily |
| ≥1.50 | 4 mg twice daily |
*The recommended dosage for patients with a BSA less than 0.40 m2 has not been established.
Missed dose: If the patient misses a dose of GOMEKLI, do not take an additional dose. Take the next scheduled dose at the prescribed time.
Vomiting: If vomiting occurs after GOMEKLI administration, do not take an additional dose. Take the next scheduled dose at the prescribed time.
2.4 Gomekli Preparation And Administration Instructions
GOMEKLI Capsules
- Swallow GOMEKLI capsules whole with or without food. If more than one capsule is required for a dose, swallow one capsule at a time.
- Do not open, break or chew capsules. Do not administer to patients who are unable to swallow a whole capsule [see GOMEKLI Tablets for Oral Suspension].
- GOMEKLI tablets for oral suspension can be swallowed whole with or without food. If more than one tablet is required for a dose, swallow one tablet at a time.
- For patients who are not able to swallow whole tablets, prepare GOMEKLI tablets for oral suspension dispersed in drinking water and administer orally as a liquid [see Instructions for Use].
- Add the prescribed number of tablets to a dosing cup containing approximately 5 mL to 10 mL of drinking water.
- Gently swirl the water and tablets until the tablets are fully dispersed and an oral suspension is obtained. It takes approximately two to four minutes to fully disperse the tablets. Once the tablets are dispersed, the oral suspension will appear white and cloudy.
- Administer the oral suspension immediately after preparation from a dosing cup or oral syringe.
- After administration of the prepared suspension, add approximately 5 mL to 10 mL of drinking water to the dosing cup and gently swirl to resuspend any remaining particles. Administer the suspension to ensure the full dose is taken.
- Discard the oral suspension if not administered within 30 minutes after preparation.
GOMEKLI Tablets for Oral Suspension
Preparation and Administration
2.5 Dosage Modifications For Adverse Reactions
The recommended dose reductions for adverse reactions are provided in Table 2.
| Body Surface Area (m2) | Reduced Dose* | |
| Morning | Evening | |
| 0.40 to 0.69 | 1 mg once daily | |
| 0.70 to 1.04 | 2 mg | 1 mg |
| 1.05 to 1.49 | 2 mg | 2 mg |
| ≥1.50 | 3 mg | 3 mg |
*Permanently discontinue GOMEKLI in patients unable to tolerate GOMEKLI after one dose reduction.
The recommended dosage modifications for adverse reactions are provided in Table 3.
| Adverse Reaction | Severity | Dosage Modification |
| Ocular Toxicity [see Warnings and Precautions (5.1)] | Grade ≤ 2 |
|
| Grade ≥ 3 |
| |
| Symptomatic Retinal Pigment Epithelium Detachment (RPED) |
| |
| Retinal Vein Occlusion (RVO) |
| |
| Left Ventricular Dysfunction [see Warnings and Precautions (5.2)] | Asymptomatic, absolute decrease in LVEF of 10% or greater from baseline and is less than the lower limit of normal |
|
| Any absolute decrease in LVEF 20% or greater from baseline |
| |
| Adverse Reaction | Severity | Dosage Modification |
| Dermatologic Adverse Reactions [see Warnings and Precautions (5.3)] | Intolerable Grade 2 or Grade 3 |
|
| Grade 3 or 4 Dermatitis Acneiform or Non-Acneiform Rash |
| |
| Other Adverse Reactions [see Adverse Reactions (6.1)] | Intolerable Grade 2 or Grade 3 |
|
| Grade 4 |
|
*Per National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (NCI CTCAE v. 5.0).
3 Dosage Forms And Strengths
Capsules:
- 1 mg: light green body and cap with “MIR 1 mg” printed on the cap in white ink.
- 2 mg: white body and a blue-green cap with “MIR 2 mg” printed on the cap in white ink.
- 1 mg: white to off-white, oval, grape flavored tablet, debossed with “S” on one side.
Tablets for Oral Suspension:
4 Contraindications
None.
5.1 Ocular Toxicity
GOMEKLI can cause ocular toxicity including retinal vein occlusion (RVO), retinal pigment epithelium detachment (RPED), and blurred vision.
In the pooled safety population [see Adverse Reactions (6.1)], ocular toxicity occurred in 25% of patients treated with GOMEKLI: 20% were Grade 1 reactions, 3.8% were Grade 2 reactions, and 0.8% were Grade 3 reactions.
Adult Patients
In the adult pooled safety population [see Adverse Reactions (6.1)], ocular toxicity occurred in 28% of patients treated with GOMEKLI: 21% were Grade 1 reactions, 5% were Grade 2 reactions and 1.3% were Grade 3 reactions. Retinal vein occlusion (RVO) occurred in 2.7% of adult patients, including one Grade 3 reaction which required permanent discontinuation of GOMEKLI. RPED occurred in one adult patient (1.3%). Blurred vision occurred in 9% of adult patients treated with GOMEKLI.
Pediatric Patients
In the pediatric pooled safety population [see Adverse Reactions (6.1)], ocular toxicity occurred in 19% of patients: 17% were Grade 1 and 1.7% were Grade 2.
Conduct comprehensive ophthalmic assessments prior to initiating GOMEKLI, at regular intervals during treatment, and to evaluate any new or worsening visual changes such as blurred vision. Continue, withhold, reduce the dose, or permanently discontinue GOMEKLI as clinically indicated [see Dosage and Administration (2.5)].
5.2 Left Ventricular Dysfunction
GOMEKLI can cause left ventricular dysfunction. Treatment with GOMEKLI has not been studied in patients with a history of clinically significant cardiac disease or LVEF <55% prior to initiation of treatment.
In the ReNeu study, in adult and pediatric patients [see Adverse Reactions (6.1)], decreased LVEF of 10 to <20% occurred in 20%, and decreased LVEF of ≥20% occurred in 0.9% of patients treated with GOMEKLI. All patients with decreased LVEF were identified during routine echocardiography. Decreased LVEF resolved in 75% of these patients.
Adult Patients
In adult patients in the ReNeu study [see Adverse Reactions (6.1)], decreased LVEF of 10 to <20% occurred in 16% of adult patients treated with GOMEKLI. Of the adult patients with decreased LVEF, five patients (9%) required dose interruption, one patient (1.7%) required a dose reduction and one patient required permanent discontinuation of GOMEKLI. The median time to first onset of decreased LVEF in adult patients was 70 days.
Pediatric Patients
In pediatric patients in the ReNeu study [see Adverse Reactions (6.1)], decreased LVEF of 10 to <20% occurred in 25%, and decreased LVEF of ≥20% occurred in 1.8% of patients treated with GOMEKLI. Of the pediatric patients with decreased LVEF, one patient (1.8%) required dose interruption of GOMEKLI. The median time to first onset of decreased LVEF in pediatric patients was 132 days.
Before initiating GOMEKLI, assess ejection fraction (EF) by echocardiogram. Monitor EF every 3 months during the first year and then as clinically indicated. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on the severity of adverse reaction [see Dosage and Administration (2.5)].
5.3 Dermatologic Adverse Reactions
GOMEKLI can cause dermatologic adverse reactions including rash.
In the pooled safety population [see Adverse Reactions (6.1)], rash occurred in 84% of patients treated with GOMEKLI: 31% were Grade 2, and 6% were Grade 3. The most frequent rashes (≥2%) included dermatitis acneiform (65%), rash (11%), eczema (8%), maculo-papular rash (4.5%) and pustular rash (3.8%).
Adult Patients
In the pooled adult safety population [see Adverse Reactions (6.1)], rash occurred in 92% of patients treated with GOMEKLI: 37% were Grade 2 and 8% were Grade 3 reactions. Rash requiring permanent discontinuation of GOMEKLI occurred in 11% of adult patients.
Pediatric Patients
In the pooled pediatric safety population [see Adverse Reactions (6.1)], rash occurred in 72% of patients treated with GOMEKLI: 22% were Grade 2 and 3.4% were Grade 3 reactions.
Rash resulting in permanent discontinuation of GOMEKLI occurred in 3.4% of pediatric patients.
Dermatitis acneiform occurred with a higher frequency in patients aged 12 to 17 years (77%) than those aged 2 to 11 years (16%), while non-acneiform rashes occurred with a higher frequency in patients aged 2 to 11 years (53%) than those aged 12 to 17 years (15%).
Initiate supportive care at first signs of dermatologic adverse reactions. Withhold, reduce the dose, or permanently discontinue GOMEKLI based on severity of adverse reaction [see Dosage and Administration (2.5)].
5.4 Embryo-Fetal Toxicity
Based on findings from clinical trials, animal studies and its mechanism of action, GOMEKLI can cause fetal harm when administered to a pregnant woman. In ReNeu, a pregnancy reported 31 days after the last dose of GOMEKLI resulted in a first trimester spontaneous abortion.
In embryo-fetal development studies, oral administration of mirdametinib to pregnant rats and rabbits during the period of organogenesis resulted in embryo-fetal mortality, structural abnormalities and alterations to growth at doses approximately equivalent to the human clinical dose of 2 mg/m2 twice daily based on body surface area (BSA).
Verify the pregnancy status of females of reproductive potential prior to the initiation of GOMEKLI. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with GOMEKLI and for 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
6 Adverse Reactions
The following serious adverse reactions are described elsewhere in the labeling:
- Ocular Toxicity [see Warnings and Precautions (5.1)]
- Left Ventricular Dysfunction [see Warnings and Precautions (5.2)]
- Dermatologic Adverse Reactions [see Warnings and Precautions (5.3)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflects exposure to GOMEKLI in 133 patients (75 adults and 58 pediatric patients) in the ReNeu study [see Clinical Studies (14)] (n=114) and Study NF-106 (n=19) [NCT-02096471].
Patients received GOMEKLI 2 mg/m2 orally twice daily for the first 21 days of each 28-day cycle until disease progression or unacceptable toxicity. Among 133 patients who received GOMEKLI, 62% were exposed for one year or longer, 38% were exposed for 2 years or longer, and 12% were exposed for 3 years or longer.
Neurofibromatosis Type 1-Associated Plexiform Neurofibromas
The safety of GOMEKLI was evaluated in the ReNeu study [see Clinical Studies (14)]. Eligible patients were 2 years of age and older with neurofibromatosis type 1 (NF1) who had symptomatic plexiform neurofibromas (PN). Patients were excluded for abnormal left ventricular ejection fraction (LVEF), uncontrolled hypertension, alanine transaminase (ALT) value of >2 × upper limit of normal (ULN), any current or history of retinal vein occlusion (RVO) or retinal pigment epithelium detachment (RPED), intraocular pressure >21 mmHg (or upper limit of normal adjusted by age), and history of glaucoma. Patients received GOMEKLI 2 mg/m2 orally twice daily for the first 21 days of each 28-day cycle until disease progression or unacceptable toxicity.
Adult Patients
The median age of adult patients (age ≥18) who received GOMEKLI was 35 years (range: 18-69); 64% were female; 85% were White, 9% were Black or African American, 3.4% were Asian, 3.4% were other races or race not reported; and 1.7% were Hispanic or Latino. For adult patients treated with GOMEKLI, the median duration of treatment was 22 months (range: 0.4 to 46 months).
Serious adverse reactions occurred in 17% of adult patients who received GOMEKLI. Serious adverse reactions occurring in ≥1% of patients were COVID-19 (3.4%), nephrolithiasis (3.4%), and in 1 patient each: acute kidney injury, abdominal pain, ischemic colitis, urinary tract infection, retinal vein occlusion, scoliosis, squamous cell carcinoma of skin, cerebrovascular accident and chronic obstructive pulmonary disease. One fatal adverse reaction occurred in an adult patient (1.7%) who received GOMEKLI, due to COVID-19.
Permanent discontinuation of GOMEKLI due to an adverse reaction occurred in 22% of adult patients. Adverse reactions which resulted in permanent discontinuation of GOMEKLI in ≥1% of adult patients were rash, diarrhea, nausea, abdominal pain, alopecia, dry skin, left ventricular dysfunction, cough, wheezing, COVID-19, peripheral swelling, RVO, dizziness, and vomiting.
Dosage interruptions of GOMEKLI due to an adverse reaction occurred in 31% of adult patients. Adverse reactions which required dosage interruption in ≥5% of patients included left ventricular dysfunction and COVID-19.
Dose reductions of GOMEKLI due to an adverse reaction occurred in 17% of adult patients. Adverse reactions which required dose reductions in ≥5% of patients included rash.
The most common adverse reactions (>25%) were rash, diarrhea, nausea, musculoskeletal pain, vomiting, and fatigue. The most common Grade 3 or 4 laboratory abnormality (>2%) was increased creatine phosphokinase.
Pediatric Patients
The median age of pediatric patients (age ≤17 years) who received GOMEKLI was 10 years (range: 2 to 17); 54% were female; 66% were White, 20% were Black or African American, 9% were other races or race not reported, 3.6% were Asian, 1.8% were American Indian or Alaska Native; and 14% were Hispanic or Latino. For pediatric patients treated with GOMEKLI, the median duration of treatment was 22 months (range: 1.6 to 40 months).
Serious adverse reactions occurred in 14% of pediatric patients who received GOMEKLI. Serious adverse reactions in ≥1% of patients included viral gastrointestinal infections (3.6%) and in 1 patient each: diplopia, musculoskeletal pain, seizure, fall, femoral neck fracture, dehydration and hypertension.
Permanent discontinuation of GOMEKLI due to an adverse reaction occurred in 9% of pediatric patients. Adverse reactions that required permanent discontinuation of GOMEKLI in ≥1% of patients were urticaria, rash, abdominal pain, constipation, and diarrhea.
Dosage interruptions of GOMEKLI due to an adverse reaction occurred in 30% of pediatric patients. Adverse reactions which required dosage interruption in ≥5% of patients included COVID-19.
Dose reductions of GOMEKLI due to an adverse reaction occurred in 13% of pediatric patients. Adverse reactions which required dosage reduction in ≥3% of pediatric patients were rash and decreased neutrophil count.
The most common adverse reactions (>25%) were rash, diarrhea, musculoskeletal pain, abdominal pain, vomiting, headache, paronychia, left ventricular dysfunction, and nausea. The most common Grade 3 or 4 laboratory abnormalities (>2%) were decreased neutrophil count and increased creatine phosphokinase.
| Adult N=58 | Pediatric N=56 | Total N=114 | ||||
| All Grades (%) | Grade 3 or 4a (%) | All Grades (%) | Grade 3 or 4a (%) | All Grades (%) | Grade 3 or 4a (%) | |
| Skin and Subcutaneous Tissue Disorders | ||||||
| Rashb | 90 | 10 | 73 | 3.6 | 82 | 7 |
| Gastrointestinal Disorders | ||||||
| Diarrheac | 59 | 0 | 55 | 5 | 57 | 2.6 |
| Nausea | 52 | 0 | 27 | 0 | 40 | 0 |
| Vomiting | 38 | 0 | 39 | 0 | 39 | 0 |
| Abdominal Paind | 24 | 3.4 | 39 | 3.6 | 32 | 3.5 |
| Stomatitise | 5 | 0 | 20 | 0 | 12 | 0 |
| Musculoskeletal and Connective Tissue Disorders | ||||||
| Musculoskeletal Painf | 41 | 5 | 41 | 1.8 | 41 | 3.5 |
| General Disorders and Administration Site Conditions | ||||||
| Fatigue | 29 | 1.7 | 13 | 0 | 21 | 0.9 |
| Pyrexia | 7 | 0 | 20 | 0 | 13 | 0 |
| Infections and Infestations | ||||||
| COVID-19g | 22 | 5 | 25 | 0 | 24 | 2.6 |
| Paronychia | 1.7 | 0 | 32 | 0 | 17 | 0 |
| Upper Respiratory Tract Infection | 0 | 0 | 23 | 0 | 11 | 0 |
| Nervous System Disorders | ||||||
| Headacheh | 14 | 1.7 | 34 | 1.8 | 24 | 1.8 |
| Peripheral Neuropathyi | 21 | 0 | 3.6 | 0 | 12 | 0 |
| Cardiac Disorders | ||||||
| Left Ventricular Dysfunction | 16 | 0 | 27 | 1.8 | 21 | 0.9 |
| Respiratory, Thoracic and Mediastinal Disorders | ||||||
| Coughj | 9 | 0 | 21 | 0 | 15 | 0 |
a All reactions were Grade 3 except one fatal case of COVID-19 in an adult.
b Rash includes dermatitis acneiform, eczema, maculo-papular rash, pustular rash, dermatitis, erythematous rash, palmar-plantar erythrodysaesthesia syndrome, exfoliative rash, skin exfoliation, pruritic rash, papule, papular rash and macular rash.
c Diarrhea includes frequent bowel movements.
d Abdominal pain includes upper abdominal pain, gastrointestinal pain and abdominal discomfort.
e Stomatitis includes mouth ulceration, aphthous ulcer.
f Musculoskeletal pain includes non-cardiac chest pain, back pain, pain in extremity, neck pain, musculoskeletal chest pain, myalgia, arthralgia, and bone pain.
g Includes one fatal case in an adult.
h Headache includes migraine.
i Peripheral neuropathy includes paresthesia, hypoesthesia, neuralgia, peripheral sensory neuropathy.
j Cough includes upper-airway cough syndrome.
Clinically relevant adverse reactions that occurred in <20% of patients include:
- Skin and Subcutaneous Tissue Disorders: alopecia, hair color changes
- Gastrointestinal Disorders: constipation
- Eye Disorders: retinal vein occlusion (RVO), retinal pigment epithelium detachment (RPED) and blurred vision
Table 5 summarizes the laboratory abnormalities in ReNeu.
| Adulta | Pediatricb | Totalc | ||||
Laboratory Abnormalityd,e | All Grades (%) | Grade 3 or 4d (%) | All Grades (%) | Grade 3 or 4d (%) | All Grades (%) | Grade 3 or 4d (%) |
| Chemistry | ||||||
| Increased Creatine Phosphokinase | 55 | 3.6 | 59 | 5 | 57 | 4.5 |
| Increased Triglycerides | 29 | 0 | 45 | 0 | 37 | 0 |
| Decreased Glucose | 5 | 0 | 36 | 1.8 | 21 | 0.9 |
| Decreased Calciumf | 23 | 0 | 20 | 0 | 21 | 0 |
| Increased Creatinine | 13 | 0 | 30 | 0 | 21 | 0 |
| Increased Cholesterol | 23 | 0 | 16 | 0 | 20 | 0 |
| Increased Alkaline Phosphatase | 13 | 0 | 29 | 0 | 21 | 0 |
| Decreased Bicarbonate | 11 | 0 | 21 | 0 | 16 | 0 |
| Increased Alanine Aminotransferase (ALT) | 9 | 0 | 21 | 0 | 15 | 0 |
| Increased Aspartate Aminotransferase (AST) | 18 | 0 | 9 | 0 | 13 | 0 |
| Hematology | ||||||
| Decreased Hemoglobin | 21 | 0 | 29 | 0 | 25 | 0 |
| Decreased Leukocytes | 7 | 0 | 40 | 0 | 23 | 0 |
| Decreased Neutrophils | 7 | 0 | 31 | 11 | 19 | 5 |
| Increased Lymphocytes | 7 | 0 | 27 | 0 | 17 | 0 |
| Decreased Lymphocytes | 16 | 0 | 1.8 | 0 | 9 | 0 |
a The denominator used to calculate the rate was 56 based on the number of patients with a baseline value and at least one post-treatment value.
b The denominator used to calculate the rate varied from 55 to 56 based on the number of patients with a
baseline value and at least one post-treatment value.
c The denominator used to calculate the rate varied from 111 to 112 based on the number of patients with a baseline value and at least one post-treatment value.
d Graded per NCI-CTCAE version 5.0.
e No Grade 5 laboratory abnormalities were reported in the ReNeu study.
f Calcium corrected for albumin (mmol/L).
8.1 Pregnancy
Risk Summary
Based on findings from clinical trials, animal studies, and its mechanism of action [see Clinical Pharmacology (12.1)], GOMEKLI can cause fetal harm or loss of pregnancy when administered to a pregnant woman. In embryo-fetal development studies, oral administration of mirdametinib to pregnant rats and rabbits during the period of organogenesis caused embryo-fetal mortality, structural abnormalities and alterations to growth at doses that were approximately equivalent to the human clinical dose of 2 mg/m2 twice daily based on BSA (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
In ReNeu, a pregnancy reported 31 days after the last dose of GOMEKLI resulted in a first trimester spontaneous abortion.
Animal Data
In an embryo-fetal developmental toxicity study, mirdametinib was administered orally to pregnant rats during the period of organogenesis (gestation days 6 to 17) at doses of 0.3, 0.6, 3 or 5 mg/kg/day. Mirdametinib caused post-implantation loss and decreased fetal body weights at doses ≥3 mg/kg/day (≥5 times the human clinical dose of 2 mg/m2 twice daily based on BSA). Multiple malformations, including shortening of limbs and absence or shortening of digits, were observed in one fetus and another with hyperflexion variation at the dose of 3 mg/kg/day.
In an embryo-fetal developmental toxicity study, mirdametinib was administered orally to pregnant rabbits during the period of organogenesis (gestation day 7 to 19) at doses of 0.3, 1, 3, or 6 mg/kg/day. Maternal toxicity (decreased body weight and moribund condition) was observed at doses ≥1 mg/kg/day (≥3 times the human clinical dose of 2 mg/m2 twice daily based on BSA). Two animals had spontaneous abortions at the 1 mg/kg dose on Days 20 and 23. Mirdametinib caused post-implantation loss at doses ≥0.3 mg/kg/day (approximately equivalent to the human clinical dose of 2 mg/m2 twice daily based on BSA).
8.2 Lactation
Risk Summary
There are no data on the presence of mirdametinib or its metabolites in human milk or their effects on a breastfed child or on milk production. Because of the potential for adverse reactions in breastfed children, advise women not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose.
8.3 Females And Males Of Reproductive Potential
GOMEKLI can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating GOMEKLI.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose.
Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with GOMEKLI and for 3 months after the last dose.
Infertility
Based on findings in animals, GOMEKLI may impair fertility in females of reproductive potential. The reversibility of the effects on female fertility in animals is unknown [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of GOMEKLI have been established in pediatric patients 2 years of age and older with NF1-PN based on the results of the ReNeu study, a single-arm trial conducted in 58 pediatric patients age ≥2 years [see Clinical Studies (14.1)]. The ReNeu study demonstrated improvement in overall response rate per REiNS criteria and duration of response.
The safety and effectiveness of GOMEKLI have not been established in pediatric patients younger than 2 years old.
Animal Toxicity Data
In a 3-month repeat-dose toxicology study in rats, oral administration of mirdametinib at doses ≥0.3 mg/kg/day (≥2 times the human exposure at the clinical dose of 2 mg/m2 twice daily based on AUC) resulted in dysplasia in femoral epiphyseal growth plate, metaphyseal hypocellularity of the bone marrow of long bones, and metaphyseal thickening of bone trabeculae of long bones; male rats were more sensitive to these effects.
8.5 Geriatric Use
Of the 133 patients with neurofibromatosis type 1 (NF1) with symptomatic plexiform neurofibromas (PN) who received GOMEKLI 2 mg/m2 orally twice daily for the first 21 days of each 28-day cycle until disease progression or unacceptable toxicity, 2 (1.5%) were 65 years of age and older and none were 75 years of age and older. Clinical studies of GOMEKLI did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently than younger adult patients.
8.6 Renal Impairment
No dosage adjustment is required in patients with mild (creatinine clearance: 60-89 mL/min) or moderate (creatinine clearance: 30-59 mL/min) renal impairment. GOMEKLI has not been studied in patients with severe (creatinine clearance <30 mL/min) renal impairment [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment is required in patients with mild hepatic impairment (total bilirubin ≤ upper limit of normal (ULN) and aspartate aminotransferase (AST) > ULN, or total bilirubin in 1-1.5 x ULN).
The pharmacokinetics of mirdametinib in patients with moderate (bilirubin >1.5 to 3 x ULN and any AST) or severe (bilirubin >3 x ULN and any AST) hepatic impairment has not been evaluated [see Clinical Pharmacology (12.3)].
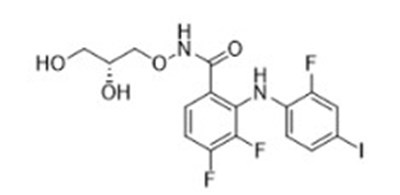
11 Description
GOMEKLI capsules and tablets for oral suspension contain mirdametinib, a kinase inhibitor. Mirdametinib is chemically known as (R)-N-(2,3-dihydroxypropoxy)-3,4-difluoro-2-((2- fluoro-4-iodophenyl)amino) benzamide. The molecular formula is C16H14 F3IN2O4 and the molecular weight is 482.20 g/mol. The structural formula for mirdametinib is:
Mirdametinib is a white to tan or pink solid with an aqueous solubility of 0.25 mg/mL and a pH of 7.2 in water at 25°C. The molecule has a pKa of 7.96.
GOMEKLI capsules and tablets for oral suspension are immediate release (IR) dosage forms intended for oral administration.
GOMEKLI (mirdametinib) 1 mg and 2 mg capsules contain 1 mg and 2 mg mirdametinib, respectively, in gelatin capsule and the following inactive ingredients: croscarmellose sodium, magnesium stearate, and microcrystalline cellulose. The gelatin capsule shell contains FD&C blue #1, gelatin, titanium dioxide, and yellow iron oxide. The capsule is imprinted with white ink that contains butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac, strong ammonia solution, and titanium dioxide.
GOMEKLI (mirdametinib) 1 mg tablets for oral suspension contain 1 mg mirdametinib and the following inactive ingredients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, grape flavor, and sucralose. The grape flavor includes corn syrup solids, modified corn starch, and triacetin.
12.1 Mechanism Of Action
Mirdametinib is an inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2). MEK1/2 proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. In vitro, mirdametinib inhibited kinase activity of MEK1 and MEK2 and downstream phosphorylation of ERK.
In a mouse model of NF1, oral dosing of mirdametinib inhibited ERK phosphorylation and reduced neurofibroma tumor volume and proliferation.
12.2 Pharmacodynamics
Mirdametinib exposure-response relationships and the time course of pharmacodynamic response are not fully characterized.
Cardiac Electrophysiology
At approximately six times the steady-state exposure associated with the recommended dose of 2 mg/m2, clinically significant QTc interval prolongation was not observed.
12.3 Pharmacokinetics
GOMEKLI pharmacokinetic parameters are summarized in Table 6.
| General Information | ||
Steady-state [mean (%CV)] | Cmax |
|
AUC |
| |
| Time to steady-state | Approximately 6 days | |
| Accumulation ratio (AUC) [mean] | 1.1 to 1.9 | |
| Absorption | ||
| Tmax [median (min, max)] |
| |
| Absolute bioavailability | No data are available in humans | |
| Food effect [GMR% (90% CI)] (high-fat, high-calorie meal) | Cmax | 57% (54%, 61%) |
| AUCinf | 93% (90%, 96%) | |
| Distribution | ||
| Human plasma protein binding | Greater than 99% | |
| Apparent volume of distribution [mean (%CV)] | 255 L (13%) | |
| Elimination | ||
| Apparent systemic clearance [mean (%CV)] | 6.3 L/h (13%) | |
| Terminal elimination half-life [mean (%CV)] | 28 h (12%) | |
| Metabolism | ||
Primary pathway | Metabolism involves glucuronidation and oxidation via UGT (primarily UGT1A6 and UGT2B7) and CES enzymes. | |
| Excretion | ||
| Radioactivity |
| |
| Unchanged mirdametinib |
| |
Abbreviations: AUC: area under the plasma concentration-time curve; AUCinf: AUC from dosing extrapolated to infinity; CI: confidence interval; CES: carboxyl esterase enzyme; Cmax: maximum plasma concentration; CV: coefficient of variation; GMR: geometric least squares mean ratio; UGT: uridine diphosphate (UDP)- glycosyltransferase
Specific Populations
Effects of Age, Sex, and Race
No clinically significant differences in mirdametinib pharmacokinetics were observed based on age (2 to 86 years), sex, and race (11% African American or Black, 12% Asian, 72% White). The effects of moderate or severe hepatic impairment, severe renal impairment, or end-stage renal disease (ESRD) on mirdametinib pharmacokinetics are unknown.
Drug Interaction Studies
No clinical DDI studies have been conducted. The effect of concomitant strong CYP3A4 inducers (that also co-induce UGTs, P-gp, and CES enzymes) on mirdametinib PK is currently unknown.
In Vitro Studies
CYP Enzymes: Mirdametinib does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4.
Mirdametinib does not induce CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP3A4.
Transporter Systems: Mirdametinib does not inhibit BCRP, P-gp, OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1, or MATE2K transporters.
Mirdametinib is a substrate of BCRP and P-gp transporters.
13.1 Carcinogenesis, Mutagenesis, Impairment Of Fertility
Mirdametinib was not carcinogenic in a 6-month study in transgenic rasH2 mice that received oral doses up to 5 mg/kg/day (approximately 15 times the human exposure at the clinical dose of 2 mg/m2 twice daily based on AUC).
Mirdametinib was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay. Mirdametinib was not clastogenic in an in vitro human lymphocyte chromosomal aberration assay or in an in vivo rat bone marrow chromosomal aberration assay. Mirdametinib was positive in the in vivo micronucleus assay in rats.
In a dedicated fertility study, male rats were treated with mirdametinib for 28 days before mating with untreated females to Gestational Day 1. Female rats were treated with mirdametinib for 14 days before mating with untreated males to Gestational Day 7. No effects on mating performance or fertility in males or females were observed at doses up to 1 mg/kg/day (approximately 2 times the human clinical dose of 2 mg/m2 twice daily based on BSA). In a 3-month repeat-dose toxicology study in rats, mirdametinib caused decreased ovarian organ weight and increased follicular cysts associated with decreases in the number of corpora lutea at doses ≥0.3 mg/kg/day (approximately 2 times the human exposure at the clinical dose of 2 mg/m2 twice daily based on AUC). Findings in male rats included hypoplasia of the spermatogenic epithelium in the testis, decreased content in the epididymis, and inflammation of the prostate at 1 mg/kg (approximately 8-times the human exposure at the clinical dose of 2 mg/m2 based on AUC). The reversibility of effects on ovary and male reproductive organs was not assessed.
14.1 Neurofibromatosis Type 1 Associated Plexiform Neurofibromas
The efficacy of GOMEKLI was evaluated in the ReNeu study (NCT03962543), a multicenter, single-arm study in 114 patients ≥2 years of age with symptomatic, inoperable neurofibromatosis type 1 (NF1) associated plexiform neurofibromas (PN) causing significant morbidity. An inoperable PN was defined as a PN that cannot be completely surgically removed without risk for substantial morbidity due to: encasement of or close proximity to vital structures, invasiveness, or high vascularity of the PN. Patients received GOMEKLI 2 mg/m2 orally twice daily for the first 21 days of each 28-day cycle until disease progression or unacceptable toxicity.
The major efficacy outcome measure was confirmed overall response rate (ORR), defined as the percentage of patients with complete response (disappearance of the target PN) or partial response (≥20% reduction in PN volume). Responses were assessed by blinded independent central review (BICR) using volumetric magnetic resonance imaging (MRI) analysis per Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) criteria modified to be confirmed at a subsequent tumor assessment within 2 to 6 months during the 24-cycle treatment phase. A secondary efficacy outcome measure was duration of response for patients who achieved a confirmed response.
Adults
A total of 58 adult patients received GOMEKLI. The median age was 35 years (range 18 to 69 years); 64% were female, 85% were White, 9% were Black or African American, 3.4% were Asian, 3.4% were other races; and 1.7% were Hispanic or Latino. Approximately half of the patients (53%) had a progressing PN at study entry, 7% had prior treatment with a MEK inhibitor and 69% had prior surgery. Morbidities reported in >25% of patients were pain (90%), disfigurement or major deformity (52%), and motor dysfunction (40%).
Pediatric Patients
A total of 56 pediatric patients received GOMEKLI. The median age was 10 years (range: 2 to 17); 54% were female; 66% were White, 20% were Black or African American, 9% were other races, 3.6% were Asian, 1.8% were American Indian or Alaska Native; and 14% were Hispanic or Latino. Most patients (63%) had a progressing PN at study entry, 11% had prior treatment with a MEK inhibitor and 36% had prior surgery. Morbidities reported in >25% of patients were pain (70%), disfigurement or major deformity (50%), and motor dysfunction (27%).
Efficacy results are provided in Table 7. The median time to onset of response was 7.8 months (range: 4 months to 19 months) for the adult cohort and 7.9 months (range: 4.1 months to 18.8 months) for the pediatric cohort.
| GOMEKLI Adult (N=58) | GOMEKLI Pediatric (N=56) | |
| Confirmed Overall Response Rate a,b,c,n (%) | 24 (41%) | 29 (52%) |
| 95% CId | (29, 55) | (38, 65) |
| Duration of Response (DoR) | ||
| DoR ≥12 monthse | 21 (88%) | 26 (90%) |
| DoR ≥24 monthse | 12 (50%) | 14 (48%) |
Abbreviations: CI: confidence interval.
a Confirmed overall response was defined as two consecutive assessments of PR or CR assessed within 2- 6 months during the Treatment Phase.
b Patients who had no post-baseline MRI assessment or no confirmed overall response were treated as non-responders.
c All partial responses.
d Obtained using the Clopper-Pearson approach.
e Duration of response was assessed based on observed time.
16 How Supplied/Storage And Handling
How supplied
GOMEKLI Capsules are supplied as follows:
| Capsule Strength | Capsule Description | Package Configuration | NDC |
| 1 mg | Light green body and cap with “MIR 1 mg” printed on the cap in white ink. | Bottle of 42 capsules | 82448-130-42 |
2 mg | White body and a blue green cap with “MIR 2 mg” printed on the cap in white ink. | Bottle of 42 capsules | 82448-260-42 |
| Bottle of 84 capsules | 82448-260-84 |
GOMEKLI Tablets for Oral Suspension are supplied as follows:
| Tablet Strength | Tablet Description | Package Configuration | NDC |
1 mg | White to off-white, oval, grape flavored tablet, debossed with “S” on one side. | Bottle of 42 tablets | 82448-134-42 |
| Bottle of 84 tablets | 82448-134-84 |
Storage and Handling
Store capsules and tablets for oral suspension at 20°C to 25°C (68°F to 77°F). Excursions permitted between 15°C to 30°C (59°F to 86°F). See USP Controlled Room Temperature. Protect from light.
17 Patient Counseling Information
Advise the patient or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Ocular Toxicity
Advise patients and caregivers that GOMEKLI can cause serious ocular effects and to immediately contact their healthcare provider if they experience any changes in their vision [see Warnings and Precautions (5.1)].
Left Ventricular Dysfunction
Advise patients and caregivers that GOMEKLI can cause decreased LVEF and to immediately report any signs or symptoms of left ventricular dysfunction to their healthcare provider [see Warnings and Precautions (5.2)].
Dermatologic Adverse Reactions
Advise patients and caregivers that GOMEKLI can cause severe dermatologic adverse reactions and to contact their healthcare provider if they experience any skin reactions [see Warnings and Precautions (5.3)]. Advise patients and caregivers that GOMEKLI can cause alopecia.
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4) and Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with GOMEKLI and for 6 weeks after the last dose [see Warnings and Precautions (5.4) and Use in Specific Populations (8.3)].
Males
Advise males with female partners of reproductive potential to use effective contraception during treatment with GOMEKLI and for 3 months after the last dose [see Warnings and Precautions (5.4) and Use in Specific Populations (8.3)].
Lactation
Advise women not to breastfeed during treatment with GOMEKLI and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential that GOMEKLI may impair fertility [see Nonclinical Toxicology (13.1)].
Administration
Advise patients to swallow GOMEKLI capsules whole (do not open, break or chew capsules) [see Dosage and Administration (2.2)].
Advise patients to either swallow GOMEKLI tablets whole or to disperse GOMEKLI tablets in water and administer orally as a liquid [see Dosage and Administration (2.2) and Instructions for Use].
Manufactured for:
SpringWorks Therapeutics, Inc. Stamford, CT 06902
GOMEKLITM is a trademark of SpringWorks Therapeutics, Inc.
©2025 SpringWorks Therapeutics, Inc.
Principal Display Panel
NDC 82448-134-84
GOMEKLITM
(mirdametinib)
Tablets for oral suspension
1 mg
per tablet
84 Tablets
NDC 82448-134-84
GOMEKLITM
(mirdametinib)
Tablets for oral suspension
1 mg
per tablet
84 Tablets
NDC 82448-134-42
GOMEKLITM
(mirdametinib)
Tablets for oral suspension
1 mg
per tablet
42 Tablets
NDC 82448-134-42
GOMEKLITM
(mirdametinib)
Tablets for oral suspension
1 mg
per tablet
42 Tablets
NDC 82448-130-42
GOMEKLITM
(mirdametinib)
Capsules
1 mg
per Capsules
42 Capsules
NDC 82448-130-42
GOMEKLITM
(mirdametinib)
Capsules
1 mg
per Capsules
42 Capsules
NDC 82448-260-42
GOMEKLITM
(mirdametinib)
Capsules
2 mg
per Capsules
42 Capsules
NDC 82448-260-42
GOMEKLITM
(mirdametinib)
Capsules
2 mg
per Capsules
42 Capsules
NDC 82448-260-84
GOMEKLITM
(mirdametinib)
Capsules
2 mg
per Capsules
84 Capsules
NDC 82448-260-84
GOMEKLITM
(mirdametinib)
Capsules
2 mg
per Capsules
84 Capsules
* Please review the disclaimer below.