Product Images Opdivo Qvantig
View Photos of Packaging, Labels & Appearance
- oq-os-checkmate-037 - image 01
- nivo-sc-carton - nivo sc carton
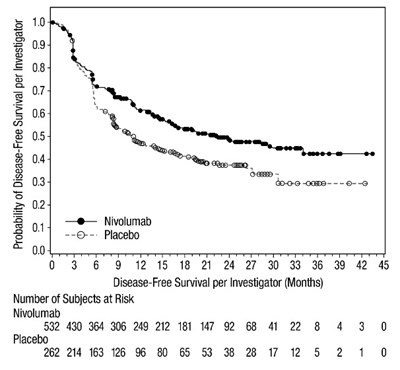
- oq-dfs-checkmate-274 - oq dfs checkmate 274
- oq-dfs-checkmate-577 - oq dfs checkmate 577
- oq-efs-checkmate-77t - oq efs checkmate 77t
- oq-efs-checkmate-816 - oq efs checkmate 816
- oq-forest-plot-os-checkmate-057 - oq forest plot os checkmate 057
- oq-forest-plot-pfs-checkmate-057 - oq forest plot pfs checkmate 057
- oq-os-attraction-3 - oq os attraction 3
- oq-os-checkmate-017 - oq os checkmate 017
- oq-os-checkmate-025 - oq os checkmate 025
- oq-os-checkmate-057 - oq os checkmate 057
- oq-os-checkmate-066 - oq os checkmate 066
- oq-os-checkmate-067 - oq os checkmate 067
- oq-os-checkmate-141 - oq os checkmate 141
- oq-os-checkmate-214 - oq os checkmate 214
- oq-os-checkmate-648a - oq os checkmate 648a
- oq-os-checkmate-648b - oq os checkmate 648b
- oq-os-checkmate-649 - oq os checkmate 649
- oq-os-checkmate-901 - oq os checkmate 901
- oq-os-pd-l1-cps-greater-than-1-checkmate-649 - oq os pd l1 cps greater than 1 checkmate 649
- oq-os-pd-l1-cps-greater-than-5-checkmate-649 - oq os pd l1 cps greater than 5 checkmate 649
- oq-pfs-checkmate-648a - oq pfs checkmate 648a
- oq-pfs-checkmate-648b - oq pfs checkmate 648b
- oq-pfs-checkmate-901 - oq pfs checkmate 901
- oq-pfs-checkmate-9er - oq pfs checkmate 9er
- oq-rfs-checkmate-238 - oq rfs checkmate 238
- oq-rfs-checkmate-76k - oq rfs checkmate 76k
- oq-updated-os-checkmate-9er - oq updated os checkmate 9er
Product Label Images
The following 29 images provide visual information about the product associated with Opdivo Qvantig NDC 0003-6120 by E.r. Squibb & Sons, L.l.c., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
oq-efs-checkmate-77t - oq efs checkmate 77t

This is a comparison of event-free survival between two treatment groups - one receiving Nivolumab with platinum-doublet chemotherapy and the other receiving Placebo with platinum-doublet chemotherapy. The data includes the number of subjects at risk over a period of 42 months. The event-free survival per blinded independent central review (BICR) is presented in months, showing the probability of survival at different time points.*
oq-efs-checkmate-816 - oq efs checkmate 816

This is a description of the Event Free Survival (EFS) per BICR (Blinded Independent Central Review) for two treatment groups: Nivolumab + platinum-doublet chemotherapy and Platinum-doublet chemotherapy. The data shows the number of subjects at risk over a span of 42 months. The EFS values are represented on the graph and indicate the survival probability over time for each treatment group.*
oq-forest-plot-os-checkmate-057 - oq forest plot os checkmate 057
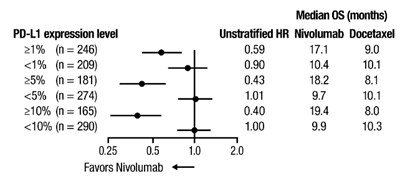
This text appears to be a statistical data table showing the comparison of different demographics based on PD-L1 expression level and their response to Nivolumab and Docetaxel treatments. The table includes patients' numbers, hazard ratios (HR), and median survival times in months. The data suggests how PD-L1 expression levels impact the efficacy of the two treatments. The table also seems to highlight that Nivolumab performs better in certain subgroups in terms of overall survival.*
oq-forest-plot-pfs-checkmate-057 - oq forest plot pfs checkmate 057
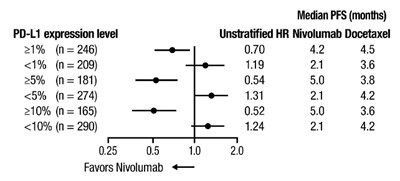
This text provides information on the median progression-free survival (PFS) in months for different PD-L1 expression levels in a study comparing the treatments Nivolumab and Docetaxel. The table shows the hazard ratio (HR) for Nivolumab compared to Docetaxel for different subgroups based on the percentage of PD-L1 expression. The data suggests that Nivolumab may provide a better median PFS compared to Docetaxel for certain PD-L1 expression levels.*
oq-os-attraction-3 - oq os attraction 3

This text describes information on Nivolumab, a drug option for cancer treatment. The data includes the probability of survival over time in months, with the number at risk for each interval provided for both Nivolumab and INV choice.*
oq-os-checkmate-025 - oq os checkmate 025

This data appears to show a comparison between two treatments, Nivolumab and Everolimus, in terms of overall survival in months. The numbers listed might represent survival rates or durations associated with each treatment. The chart seems to display a trend of survival over time for both treatments.*
oq-os-checkmate-066 - oq os checkmate 066

This text appears to be a chart showing data related to a study on Survival. It includes values for Nivolumab and Dacarbazine over a period of months. Nivolumab data ranges from 210 to 0 months, while Dacarbazine data ranges from 208 to 0 months. The chart seems to have multiple intervals and values which were compared for the two different treatments.*
oq-os-checkmate-067 - oq os checkmate 067

This information shows a comparison of three different treatments (Nivolumab, Nivolumab + Ipilimumab, and Ipilimumab) in terms of overall survival probability. The data includes the number of subjects at risk over time, up to 30 months, for each treatment. The graph displays the survival rate over the months for each treatment option.*
oq-os-checkmate-141 - oq os checkmate 141

This is a survival analysis for the drug Nivolumab compared to the investigator's choice, based on the overall survival rate over a period of 18 months. The graph shows the number of participants at risk for each treatment group at different time points during the study.*
oq-os-checkmate-214 - oq os checkmate 214

This is a report on the probability of overall survival for two treatment groups: nivolumab + ipilimumab and sunitinib. The data includes the number of subjects at risk and their overall survival in months. Nivolumab + ipilimumab had 21 subjects at risk, with survival times of 45, 39, 37, and more months. Sunitinib had 42 subjects at risk, with survival times ranging from 42 to 19 months.*
oq-os-checkmate-648b - oq os checkmate 648b

This text shows a comparison of overall survival rates for different treatment options including Nivolumab + ipilimumab, Nivolumab + chemotherapy, and Chemotherapy. The data provided includes the number of subjects at risk over time and overall survival months for each treatment group.*
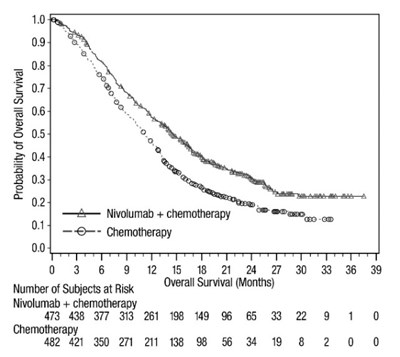
oq-os-checkmate-649 - oq os checkmate 649

This data shows the comparison of overall survival in a study evaluating the combination of Nivolumab and chemotherapy versus chemotherapy alone. The number of subjects at risk is listed at different time points, ranging from 0 to 39 months. The Nivolumab + chemotherapy group starts with 789 subjects and decreases over time, while the chemotherapy group starts with 792 subjects. This information can be used to analyze the effectiveness of the treatment combination.*
oq-os-checkmate-901 - oq os checkmate 901

The text provides data on the probability of overall survival for two treatment groups: Nivolumab with gemcitabine + cisplatin and Gemcitabine + cisplatin. The survival data is presented in months (from 0 to 63 months) with the number of subjects at risk at each time point listed for both treatment groups. This information can be used to analyze and compare the overall survival outcomes between the two treatments.*
oq-os-pd-l1-cps-greater-than-5-checkmate-649 - oq os pd l1 cps greater than 5 checkmate 649
This text appears to be a table containing data related to a study comparing nivolumab + chemotherapy with chemotherapy alone. The table includes the number of subjects at risk, survival data in months, and endpoints related to the study.*
oq-pfs-checkmate-648a - oq pfs checkmate 648a

This document seems to be related to a clinical study comparing the effectiveness of different treatments for Progression Free Survival. The treatments studied include Nivolumab + ipilimumab, Nivolumab + chemotherapy, and chemotherapy alone. The text also mentions the number of subjects at risk over time for each treatment group. It appears to present data on the Progression Free Survival per BICR (Blinded Independent Central Review) in months for each treatment group.*
oq-pfs-checkmate-648b - oq pfs checkmate 648b

This text provides information about different treatment combinations for Progression Free Survival in a study. The treatments evaluated are Nivolumab + ipilimumab, Nivolumab + chemotherapy, and Chemotherapy. The chart shows the progression-free survival data over months for each treatment combination along with the number of subjects at risk at different time points.*
oq-pfs-checkmate-901 - oq pfs checkmate 901

This document presents the Probability of Progression Free Survival per BICR for two different treatments over time. The treatments compared are Nivolumab with gemcitabine+cisplatin and Gemcitabine+cisplatin. The data includes percentages ranging from 0% to 90%, as well as a timeline showing the months of treatment progression and the number of subjects at risk at different time points.*
oq-pfs-checkmate-9er - oq pfs checkmate 9er

This is a comparison of the progression-free survival probabilities between treatments of Nivolumab + Cabozantinib and Sunitinib. The data shows the time points at 3, 6, and 9 months with the number of subjects at risk for each treatment. For Nivolumab + Cabozantinib, there are 33 subjects at the start and decreasing numbers as time progresses (279 at 3 months, 84 at 6 months, and so on). This information can be valuable for evaluating the effectiveness of these treatments in terms of progression-free survival.*
oq-updated-os-checkmate-9er - oq updated os checkmate 9er
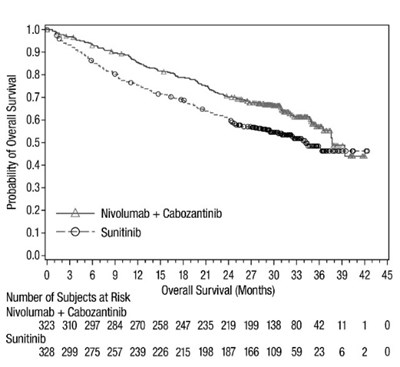
The provided text shows data related to the probability of overall survival for patients receiving Nivolumab + Cabozantinib and Sunitinib treatments. The graph displays the number of subjects at risk over different months of overall survival. The data points indicate the number of subjects remaining at each time interval, showcasing the survival trend for each treatment group.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.