Product Images Glatiramer Acetate
View Photos of Packaging, Labels & Appearance
- Glatiramer Acetate Structural Formula - 5cd25695 8a5c 48b6 bd73 86a48de41493 01
- Figure 1: Time to Second Exacerbation - 5cd25695 8a5c 48b6 bd73 86a48de41493 02
- Figure 2: Median Cumulative Number of Gd-Enhancing Lesions - 5cd25695 8a5c 48b6 bd73 86a48de41493 03
- Instructions for Use Figure A - image 01
- Instructions for Use Figure B - image 02
- Instructions for Use Figure C - image 03
- Instructions for Use Figure D - image 04
- Instructions for Use Figure E - image 05
- Instructions for Use Figure F - image 06
- Instructions for Use Figure G - image 07
- Instructions for Use Figure H - image 08
- Instructions for Use Figure I - image 09
- Instructions for Use Figure J - image 10
- Instructions for Use Figure K - image 11
- Instructions for Use Figure L - image 12
- Glatiramer Acetate Injection 40 mg/mL Carton Label - image 13
Product Label Images
The following 16 images provide visual information about the product associated with Glatiramer Acetate NDC 0378-6961 by Mylan Pharmaceuticals Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 1: Time to Second Exacerbation - 5cd25695 8a5c 48b6 bd73 86a48de41493 02

This appears to be a statistical analysis report of Glatiramer Acetate, a medication used to treat multiple sclerosis. The hazard ratio is given as 0.55 which indicates a lower hazard rate in patients taking the drug compared to placebo. The table shows the percentage of patients experiencing a second exacerbation on placebo vs Glatiramer Acetate over a period of 10 days. However, some of the text is corrupted and the table format is unclear, so the information may not be entirely usable.*
Figure 2: Median Cumulative Number of Gd-Enhancing Lesions - 5cd25695 8a5c 48b6 bd73 86a48de41493 03

Instructions for Use Figure C - image 03
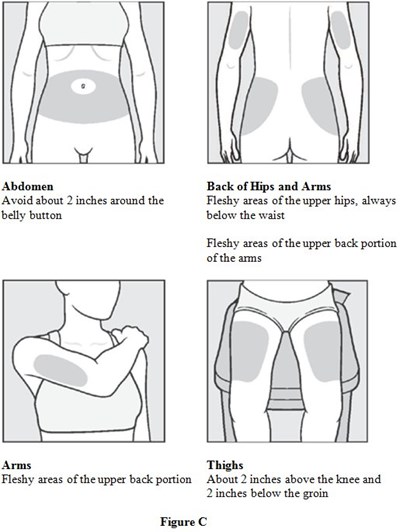
This appears to be a set of instructions for body measurement, with specific areas to avoid and areas to measure for the abdomen, back of hips and arms, arms, and thighs. The text refers to fleshy areas and provides measurements for each area. There is also an accompanying image labeled "Figure C".*
Glatiramer Acetate Injection 40 mg/mL Carton Label - image 13

This is a prescription drug called Glatiramer Acetate. It is an injection given under the skin, and it should be refrigerated and protected from light. It is available in 40mg/mL strength, and the usual dosage is three times a week. The medication comes in pre-filled syringes with fixed needles. The package contains 12 syringes and the prescription needs to be dispensed as a unit package in a carton. The active ingredient is 40mg of Glatiramer Acetate, and the inactive ingredients are manitol and sterile water. Dosage and other important information can be found in the accompanying prescribing information.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.











