FDA Label for Satohap Lidocaine 4% Menthol 1% Pain Relieving Cream
View Indications, Usage & Precautions
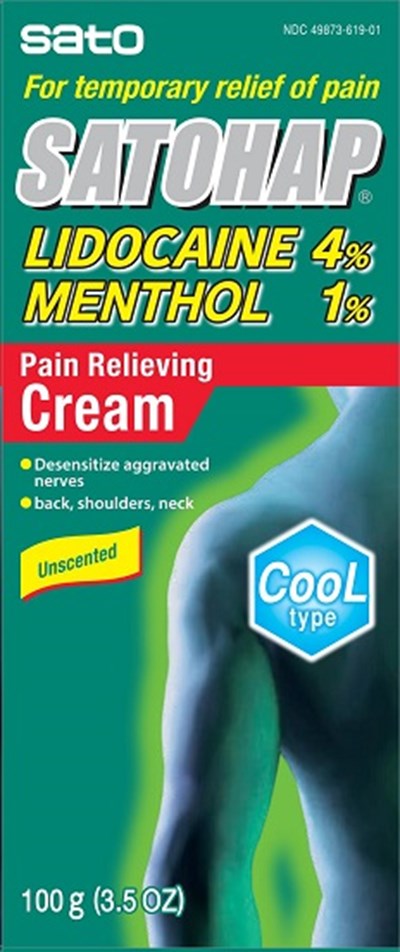
Satohap Lidocaine 4% Menthol 1% Pain Relieving Cream Product Label
The following document was submitted to the FDA by the labeler of this product Sato Pharmaceutical Co., Ltd.. The document includes published materials associated whith this product with the essential scientific information about this product as well as other prescribing information. Product labels may durg indications and usage, generic names, contraindications, active ingredients, strength dosage, routes of administration, appearance, warnings, inactive ingredients, etc.
Otc - Active Ingredient
Active ingredients
Lidocaine 4%
Menthol 1%
Otc - Purpose
Purpose
Lidocaine Topical analgesic
Menthol Topical analgesic
Indications & Usage
Uses
For temporary relief of pain
Warnings
Warnings
For external use only
Otc - Do Not Use
Do not use in large quantities, particularly over raw surfaces or blistered areas
Otc - When Using
When using this product avoid contact with the eyes
Otc - Stop Use
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- a rash or irritation develops
Otc - Keep Out Of Reach Of Children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Dosage & Administration
Directions
- adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times dailly
- children under 12 years of age: do not use, ask a doctor
Other Safety Information
Other information
- protect product from excessive moisture
- avoid storing in direct sunlight
- store with lid tightly closed
Inactive Ingredient
Inactive ingredients benzyl alcohol, butylene glycol, carbomer homopolymer (type C), isopropyl myristate, polyoxyl 40 hydrogenated castor oil, sodium hydroxide, water
Package Label.Principal Display Panel
* Please review the disclaimer below.