Product Images Xarelto
View Photos of Packaging, Labels & Appearance
- ce538950 c1cf 4b9b 9434 a4b96c1d461e 00
- ce538950 c1cf 4b9b 9434 a4b96c1d461e 01
- ce538950 c1cf 4b9b 9434 a4b96c1d461e 02
- Figure 1 - xarelto 01
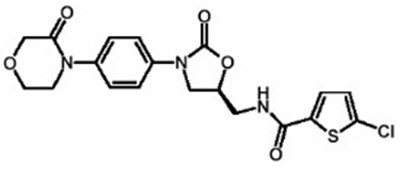
- Chemical Structure - xarelto 02
- Figure 2 - xarelto 03
- Figure 3 - xarelto 04
- Figure 4 - xarelto 05
- Figure 5 - xarelto 06
- Figure 6 - xarelto 07
- Figure 7 - xarelto 08
- Figure 8 - xarelto 09
- xarelto 10
- xarelto 11
- xarelto 12
- xarelto 13
Product Label Images
The following 16 images provide visual information about the product associated with Xarelto NDC 55154-1424 by Cardinal Health 107, Llc, such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
ce538950 c1cf 4b9b 9434 a4b96c1d461e 00

This is a medicine package for a product made by Janssen Pharmaceuticals Inc. that includes an active ingredient made in Germany. The medicine is distributed by CardinalHealth and has an NDC number of 50458 579-50. The manufacturer is licensed by Bayer Healthcare AG. The rest of the text is not legible.*
ce538950 c1cf 4b9b 9434 a4b96c1d461e 02

XARELTO is a medication package containing 10 tablets of Rivaroxaban, each tablet having 10 mg of the active ingredient. This medication is used to prevent blood clots in various medical conditions. It is advised to store this package at room temperature, and it may not be child-resistant. This particular package is intended for hospital use only and is Rx only. A medication guide should be dispensed to each patient. It is manufactured in Germany and licensed from Bayer Healthcare AG, then marketed by Janssen Pharmaceuticals, Inc., and distributed by Cardinal Health. Refer to the product insert for complete prescribing information, precautions, and warnings.*
Figure 3 - xarelto 04

This is a table that lists different types of interacting drugs with their PK (pharmacokinetic) fold change and 90% confidence interval. The drugs are categorized into combined P-gp and strong or moderate CYP3A inhibitors or inducers, as well as moderate CYP3A inhibitors. The names of the drugs listed are Clpeomyen, Epromyen, Fconazde, and "other drugs".*
Figure 4 - xarelto 05

XARELTO is a drug name, but the rest of the text seems to be numerical data and not a useful description.*
xarelto 12

This appears to be a graph or chart displaying the cumulative incidence rate (%) over time (measured in days from randomization) for two groups - one treated with XARELTO and one treated with a placebo. It provides the number of events (either cases or individuals) in each group at different time points. However, without further context or information, the purpose or meaning of this data is not clear.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.