Product Images Sovaldi
View Photos of Packaging, Labels & Appearance
- Chemical Structure - sovaldi 01
- sovaldi 01a
- sovaldi 01b
- sovaldi 01c
- sovaldi 01d
- sovaldi 01e
- sovaldi 01f
- sovaldi 01g
- sovaldi 01h
- sovaldi 01i
- sovaldi 01j
- sovaldi 01k
- sovaldi 01l
- sovaldi 01m
- sovaldi 01n
- PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle Label - sovaldi 02
- PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle Label - sovaldi 03
- sovaldi 04
- sovaldi 05
- sovaldi 06
Product Label Images
The following 20 images provide visual information about the product associated with Sovaldi NDC 61958-1503 by Gilead Sciences, Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle Label - sovaldi 02

This is a description of Sovaldi, a medication manufactured by Gilead Sciences, Inc. Each tablet contains 400 mg of sofosbuvir and is dispensed in its original container with a unique NDC number. The medication is taken once daily according to the dosage and administration instructions included in the package insert. The container carries an "ALERT" box that should not be covered by the pharmacy label. The text does not contain any further information.*
PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle Label - sovaldi 03
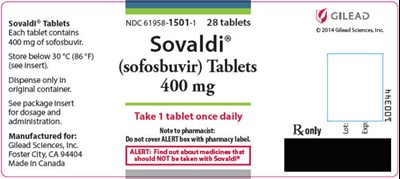
Sovaldi® Tablets are a product of Gilead Sciences. The Tablets come in a bottle containing 28 tablets with each tablet containing 400 mg of sofosbuvir. The tablets should be stored below 30 °C (86 °F). The package contains an insert giving dosage and administration advice. The recommended dosage is one tablet per day. These tablets should only be dispensed in their original container. The product is manufactured for Gilead Sciences, Inc. in Canada.*
sovaldi 04
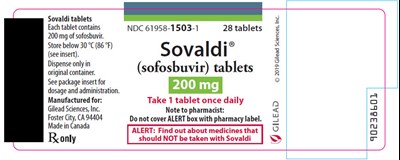
Sovaldi tablets contain 200mg of sofosbuvir each and must be stored below 30 °C (86 °F). Dosage and administration information can be found in the package insert. These tablets are manufactured for Gilead Sciences, Inc. and made in Canada. Each package contains 28 tablets and should only be dispensed in the original container. The recommended dosage is one tablet per day. The ALERT box on the package should not be covered by a pharmacy label. There is some unclear text which may indicate the medicine should not be taken with other drugs, but it is not fully legible.*
sovaldi 05

Sovaldi® is a medication available in the form of oral pellets. Each packet contains 150mg of the medication. There are 28 packets in one container identified by the NDC code 61958-1504-1. The package includes an alert box for informing patients about medicine combinations that should be avoided while taking Sovaldi®.*
sovaldi 06
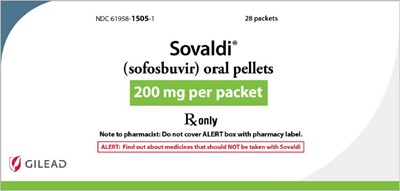
This is a packaging of Sovaldi® (sofosbuvir) oral pellets with 28 packets, each containing 200 mg. The labeled box also contains a note to pharmacists to not cover the ALERT box with the pharmacy label. The second half of the text appears to be cut off and is not available.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.














