Product Images Hercessi
View Photos of Packaging, Labels & Appearance
- Figure 1 - hercessi 01
- Figure 2 - hercessi 02
- Figure 3 - hercessi 03
- Figure 4 - hercessi 04
- Figure 5 - hercessi 05
- Figure 6 - hercessi 06
- Figure 7 - hercessi 07
- PRINCIPAL DISPLAY PANEL - 150 mg Vial Carton - hercessi 08
- PRINCIPAL DISPLAY PANEL - 150 mg Vial Carton - hercessi 09
- PRINCIPAL DISPLAY PANEL - 420 mg Vial Carton - hercessi 10
- PRINCIPAL DISPLAY PANEL - 420 mg Vial Carton - hercessi 11
Product Label Images
The following 11 images provide visual information about the product associated with Hercessi NDC 69448-015 by Accord Biopharma Inc., such as packaging, labeling, and the appearance of the drug itself. This resource could be helpful for medical professionals, pharmacists, and patients seeking to verify medication information and ensure they have the correct product.
Figure 6 - hercessi 06
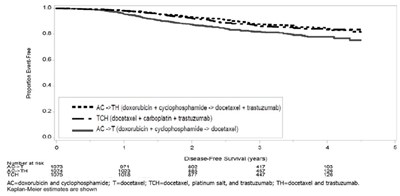
This text provides information regarding different treatment regimens for a specific type of disease, including combinations such as doxorubicin + cyclophosphamide, docetaxel + carboplatin + trastuzumab, and more. It also mentions disease-free survival data over a period of years. Additionally, the text includes definitions for the abbreviations AC, T, TCH, and TH. It also refers to Kaplan-Meier estimates being shown.*
PRINCIPAL DISPLAY PANEL - 150 mg Vial Carton - hercessi 08

This text provides information about a medication called "HERCESSI" (trastuzumab-strf), an injection with a dosage of 150 mg per vial. It mentions that it is a single-dose vial and instructs to discard any unused solution and not freeze it. The medication is intended for intravenous infusion and should not be shaken after reconstitution. It also advises to store it at 2°C-8°C and protect it from light until the time of reconstitution. The text includes the NDC number for the product and the manufacturer as Accord BioPharma Inc. It gives a directive to reconstitute immediately before use.*
PRINCIPAL DISPLAY PANEL - 420 mg Vial Carton - hercessi 11

This text provides detailed instructions and information about "HERCESSI" (trastuzumab-strf) for injection. The product comes in a 420 mg per vial multiple-dose form and is intended for intravenous infusion after reconstitution. The reconstitution process involves using 20 mL of Bacteriostatic Water for Injection. It is crucial to store the product at 2°C to 8°C until reconstitution, avoid freezing, and protect it from light. The manufacturer is Accord BioPharma Inc., and the product details also include a National Drug Code (NDC) and other technical information. The recommended dosage and additional details can be found in the Prescribing Information.*
* The product label images have been analyzed using a combination of traditional computing and machine learning techniques. It should be noted that the descriptions provided may not be entirely accurate as they are experimental in nature. Use the information in this page at your own discretion and risk.







